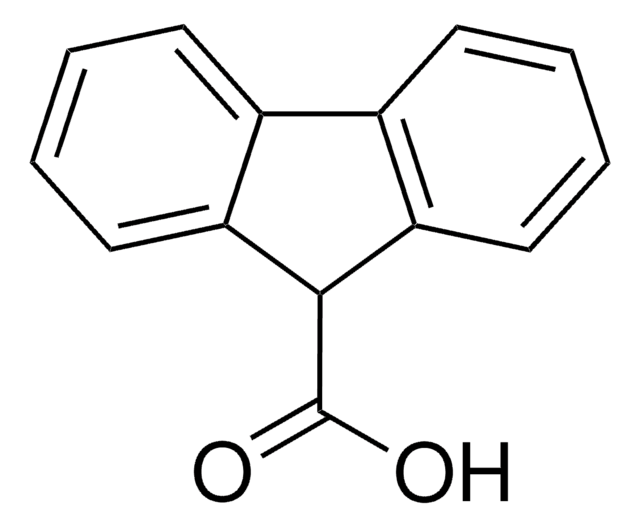
A89405
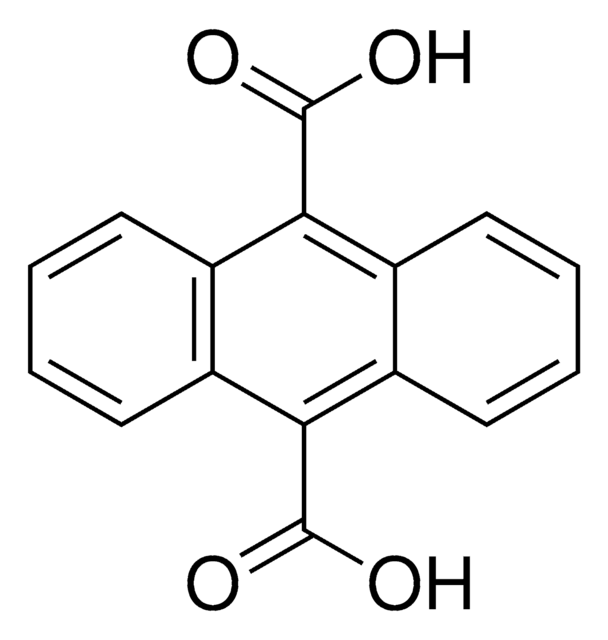
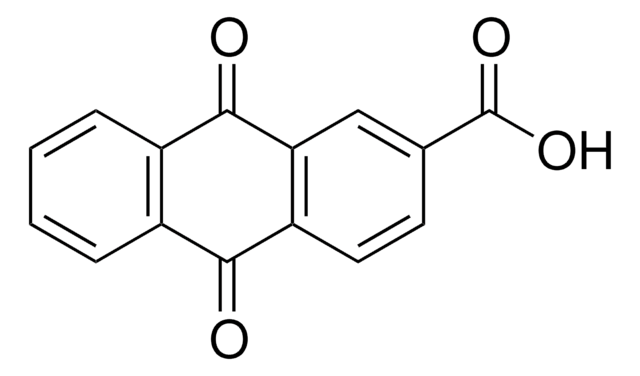
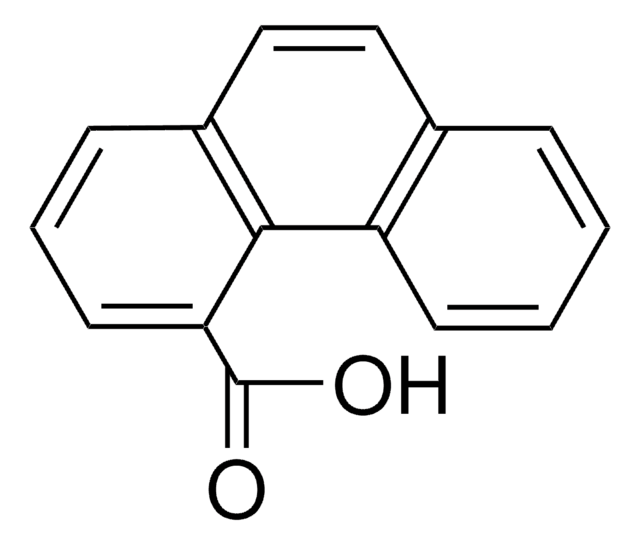
9-Anthracenecarboxylic acid
99%
Sinonimo/i:
9-Anthroic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C15H10O2
Numero CAS:
Peso molecolare:
222.24
Beilstein:
1875336
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Stato
powder
Punto di fusione
213-217 °C (lit.)
λmax
254 nm at 0.1% in ethanol
Stringa SMILE
OC(=O)c1c2ccccc2cc3ccccc13
InChI
1S/C15H10O2/c16-15(17)14-12-7-3-1-5-10(12)9-11-6-2-4-8-13(11)14/h1-9H,(H,16,17)
XGWFJBFNAQHLEF-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
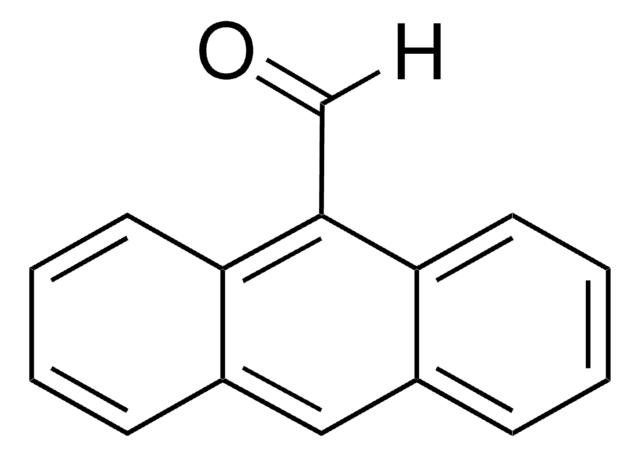
9-Anthracenecarboxylic acid can be used as a starting material to synthesize:
It can also be used as a cross-linking agent to functionalize organic second-order nonlinear optical materials to enhance poling efficiency and temporal stability.
- 9-Cyanoanthracene by reacting with cyanohydrins in the presence of a palladium catalyst.
- 6-Chloro-8-(9-anthracenyl)-9H-purine by Ag/SiO2 catalyzed reaction one-pot reaction with 6-chloro-4,5-pyrimidinediamine.
It can also be used as a cross-linking agent to functionalize organic second-order nonlinear optical materials to enhance poling efficiency and temporal stability.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Krisztina Váczi et al.
Naunyn-Schmiedeberg's archives of pharmacology, 388(1), 87-100 (2014-10-26)
Understanding the role of ionic currents in shaping the cardiac action potential (AP) has great importance as channel malfunctions can lead to sudden cardiac death by inducing arrhythmias. Therefore, researchers frequently use inhibitors to selectively block a certain ion channel
Straightforward conversion of arene carboxylic acids into aryl nitriles by palladium-catalyzed decarboxylative cyanation reaction
Ouchaou K, et al.
Synlett, 2010(14), 2083-2086 (2010)
Mild and in situ photo-crosslinking of anthracene-functionalized poly (aryl ether ketone) for enhancing temporal stability of organic NLO materials
Tian Yanxin, et al.
J. Mater. Sci., 56(9), 5910-5923 (2021)
Bence Hegyi et al.
Journal of molecular and cellular cardiology, 109, 27-37 (2017-07-03)
The role of Ca2+-activated Cl- current (ICl(Ca)) in cardiac arrhythmias is still controversial. It can generate delayed afterdepolarizations in Ca2+-overloaded cells while in other studies incidence of early afterdepolarization (EAD) was reduced by ICl(Ca). Therefore our goal was to examine
Ag Loaded on SiO2 as an Efficient and Recyclable Heterogeneous Catalyst for the Synthesis of Chloro-8-substituted-9H-purines
Maddila S, et al.
Journal of Heterocyclic Chemistry, 53(1), 319-324 (2016)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| A89405-10G | |
| A89405-25G | 4061833400968 |
| A89405-500G | |
| A89405-5G | 4061831832501 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.