180246
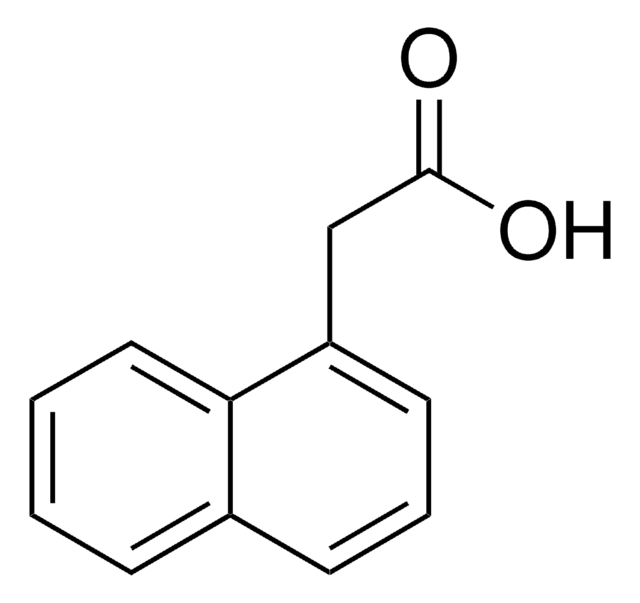
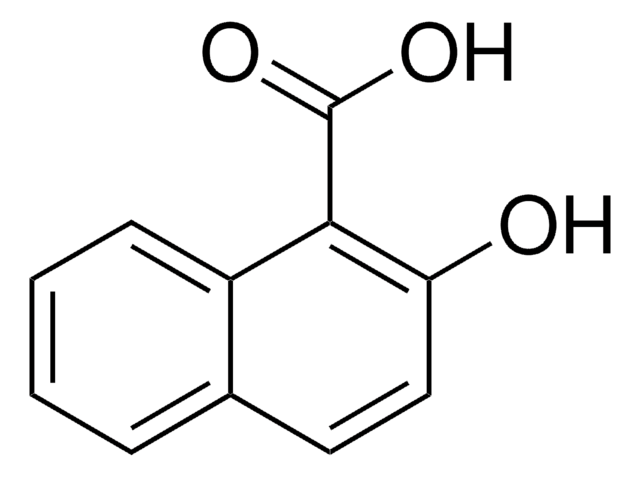
2-Naphthoic acid
98%
Sinonimo/i:
2-Naphthalenecarboxylic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C10H7CO2H
Numero CAS:
Peso molecolare:
172.18
Beilstein:
972039
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Forma fisica
powder
Punto di fusione
185-187 °C (lit.)
Solubilità
alcohol: soluble
diethyl ether: soluble
hot water: slightly soluble
Stringa SMILE
OC(=O)c1ccc2ccccc2c1
InChI
1S/C11H8O2/c12-11(13)10-6-5-8-3-1-2-4-9(8)7-10/h1-7H,(H,12,13)
UOBYKYZJUGYBDK-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
2-Naphthoic acid (NPA) is a noncompetitive N-methyl-D-aspartate (NMDA) receptor inhibitor. The fluorescence spectra and electronic absorption of 2-naphthoic acid was studied.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
An electronic spectral study of the influence of thermal and electronic processes on the determination of the excited singlet-state dissociation constants of 1-and 2-naphthoic acid.
Kovi PJ and Schulman SG.
Analytica Chimica Acta, 63(1), 39-52 (1973)
Han Yu et al.
Molecular pharmacology, 84(4), 541-550 (2013-07-23)
N-Methyl-D-aspartate (NMDA) receptors mediate excitatory synaptic transmission in the central nervous system and play important roles in synaptic development and plasticity, but also mediate glutamate neurotoxicity. Recently, 2-naphthoic acid (NPA) and its derivatives have been identified as allosteric, noncompetitive NMDA
V Krishnakumar et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 70(1), 201-209 (2007-09-08)
The solid phase mid FTIR and FT Raman spectra of 2-naphthoic acid (NA) and 6-bromo-2-naphthoic acid (BNA) have been recorded in the regions 4000-400 cm(-1) and 3500-100 cm(-1), respectively. The fundamental vibrational frequencies and intensities of the vibrational bands were
M J Melancon et al.
Drug metabolism and disposition: the biological fate of chemicals, 10(2), 128-133 (1982-03-01)
Urine was collected from four female rats for 3 days after two subcutaneous injections with 0.3 mg of 2-methyl[8-14C]naphthalene per kg. Of the 14C injected, 55% was found in the urine. The urine was solvent-fractionated into a toluene fraction (4.9%
Blaise Mathias Costa et al.
Neuropharmacology, 62(4), 1730-1736 (2011-12-14)
Over-activation of N-methyl-d-aspartate (NMDA) receptors is critically involved in many neurological conditions, thus there has been considerable interest in developing NMDA receptor antagonists. We have recently identified a series of naphthoic and phenanthroic acid compounds that allosterically modulate NMDA receptors
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.