133760
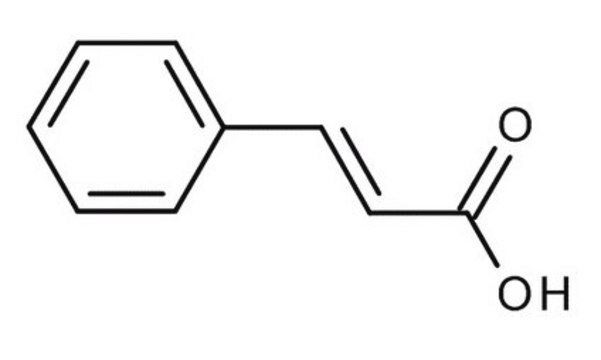
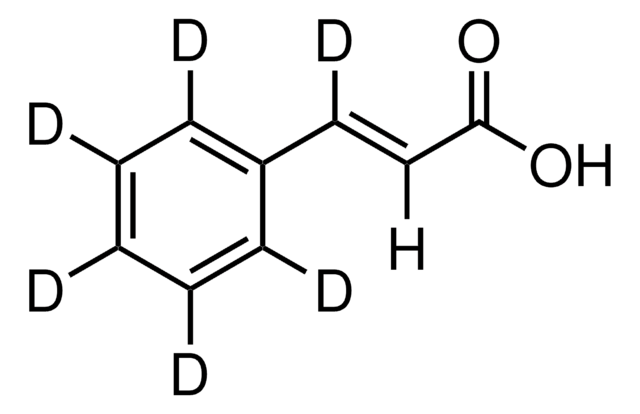
trans-Cinnamic acid
97%
Sinonimo/i:
trans-3-Phenylacrylic acid, Cinnamic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5CH=CHCOOH
Numero CAS:
Peso molecolare:
148.16
Beilstein:
1905952
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
eCl@ss:
39023931
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Stato
solid
P. ebollizione
300 °C (lit.)
Punto di fusione
132-135 °C (lit.)
Gruppo funzionale
carboxylic acid
phenyl
Stringa SMILE
OC(=O)\C=C\c1ccccc1
InChI
1S/C9H8O2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H,(H,10,11)/b7-6+
WBYWAXJHAXSJNI-VOTSOKGWSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
trans-Cinnamic acid was used to establish library of phenolic compounds by liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry.
Azioni biochim/fisiol
trans-cinnamic acid has inhibitory effect on phorbol-12-myristate-13-acetate-induced invasion of human lung adenocarcinoma A549 cells. It is a potential agent which can prevent lung tumor cells from metastasizing. It induces intracellular release of Ca2+ from the vacuole to the cytoplasm which triggers phytotoxicity in cucumber.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
320.0 °F - closed cup
Punto d’infiammabilità (°C)
160 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Jianping Sun et al.
Molecules (Basel, Switzerland), 12(3), 679-693 (2007-09-14)
Liquid chromatography/ultraviolet (LC/UV) and mass spectrometry/mass spectrometry (MS/MS) libraries containing 39 phenolic compounds were established by coupling a LC and an ion trap MS with an electrospray ionization (ESI) source, operated in negative ion mode. As a result, the deprotonated
Chiung-Man Tsai et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 48(3), 494-501 (2012-12-12)
Dietary polyphenols have been reported as an effective phytochemical for health protection and cinnamic acid (CA) is one of the polyphenols that has been demonstrated having chemopreventive potential. It was known that the early and distal metastasis might lead to
Jingquan Yu et al.
Journal of chemical ecology, 35(12), 1471-1477 (2010-01-12)
To obtain insight into interspecies interactions mediated by allelochemicals, the response of cucumber (Cucumis sativus L. cv Jinyan No.4) and figleaf gourd (Cucurbita ficifolia Bouché) seedlings to trans-cinnamic acid (CA) (1) was investigated. While trans-CA is an autotoxin in cucumber
Feng Yang et al.
Molecular pharmaceutics, 9(11), 3259-3265 (2012-09-27)
Owing to advantageous biochemical and pharmacological properties of human serum albumin (HSA), HSA-based drug carrier is playing an increasing role in the clinical setting. Since the IIA subdomain of HSA is a big hydrophobic cavity, we proposed that HSA delivers
Andrew M Lauer et al.
Organic letters, 14(19), 5138-5141 (2012-09-25)
A highly regioselective, Pd-catalyzed allylic fluorination of phosphorothioate esters is reported. This chemistry addresses several limitations of previously reported methods in which elimination and lack of reactivity were problematic. Preliminary mechanistic investigations reveal that these reactions are stereospecific and provide
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.