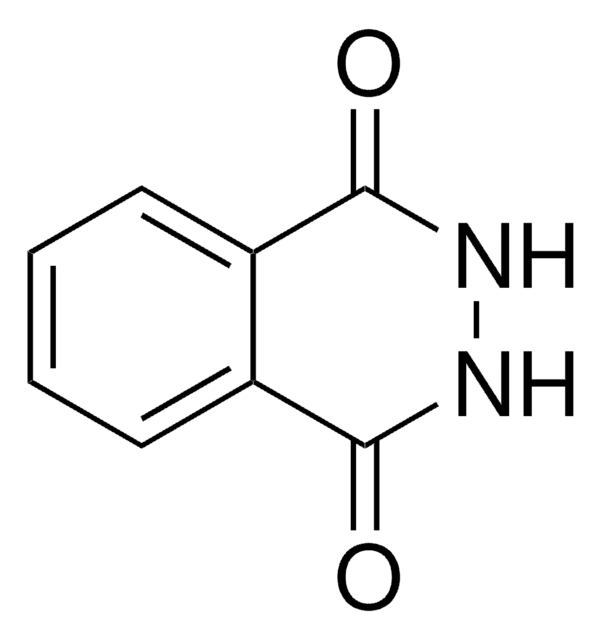
131296
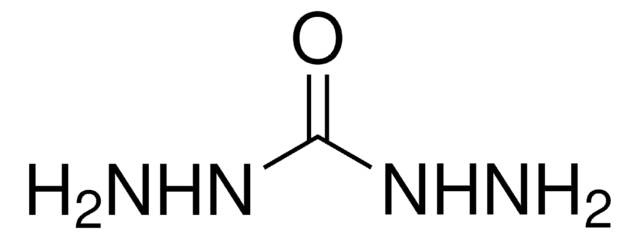
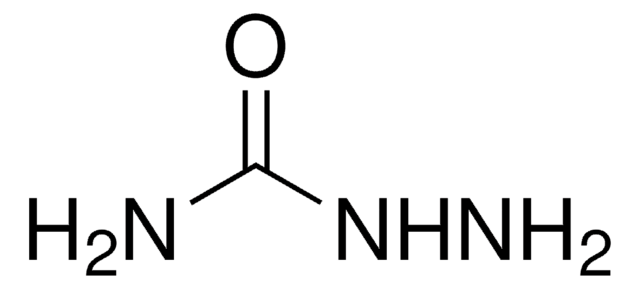
Oxalyldihydrazide
98%
Sinonimo/i:
Oxalic dihydrazide, Oxalylhydrazide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
NH2NHCOCONHNH2
Numero CAS:
Peso molecolare:
118.09
Beilstein:
1072110
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Punto di fusione
242-244 °C (dec.) (lit.)
Stringa SMILE
NNC(=O)C(=O)NN
InChI
1S/C2H6N4O2/c3-5-1(7)2(8)6-4/h3-4H2,(H,5,7)(H,6,8)
SWRGUMCEJHQWEE-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Oxalyldihydrazide was used as a crosslinker for enrichment of carbonylated proteins within a microfluid chip. It was also used to synthesize a new series of manganese and iron salt forming complexes by template condensation with glyoxal.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
M Rizk et al.
Acta pharmaceutica Hungarica, 62(4), 158-166 (1992-07-01)
A sensitive and selective colorimetric method has been devised for the micro-determination of copper (II) ions in pure form, some chemicals and multivitamin preparations. The method depends on the formation of stable blue complexes in ammonia medium peaking at 615
Khlood Abou Melha
Journal of enzyme inhibition and medicinal chemistry, 23(2), 285-295 (2008-03-18)
The Schiff base ligand, oxalyl [( 2 - hydroxybenzylidene) hydrazone] [corrected].H(2)L, and its Cu(II), Ni(II), Co(II), UO(2)(VI) and Fe(III) complexes were prepared and tested as antibacterial agents. The Schiff base acts as a dibasic tetra- or hexadentate ligand with metal
Bryant C Hollins et al.
Lab on a chip, 12(14), 2526-2532 (2012-05-09)
We report a proof of principle study for the use of oxalyldihydrazide as a crosslinker for enrichment of carbonylated proteins within a microfluidic chip. Surface modification steps are characterized and analyzed using analytical techniques. We use oxidized cytochrome c as
D P Singh et al.
Journal of enzyme inhibition and medicinal chemistry, 24(3), 883-889 (2008-10-29)
A new series of complexes is synthesized by template condensation of oxalyldihydrazide and glyoxal in methanolic medium in the presence of trivalent chromium, manganese and iron salts forming complexes of the type: [M(C(8)H(8)N(8)O(4))X]X(2) where M = Cr(III), Mn(III), Fe(III) and
Zahid H Chohan et al.
Journal of enzyme inhibition and medicinal chemistry, 17(1), 1-7 (2002-10-09)
Schiff bases derived from oxaldiamide/oxalylhydrazine and pyrrol-2-carbaldehyde, or salicylaldehyde respectively, as well as their Zn(II) complexes have been prepared and tested as antibacterial agents. These Schiff bases function as tetradentate ligands, forming octahedral Zn(II) complexes. The ketonic form for the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.