122351
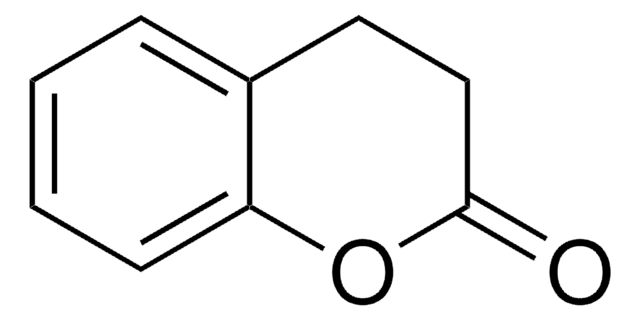
4-Chromanone
97%
Sinonimo/i:
2,3-Dihydro-1-benzopyran-4-one
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9H8O2
Numero CAS:
Peso molecolare:
148.16
Beilstein:
124652
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
solid
Indice di rifrazione
n20/D 1.575 (lit.)
P. ebollizione
127-128 °C/13 mmHg (lit.)
Punto di fusione
35-38 °C (lit.)
Stringa SMILE
O=C1CCOc2ccccc12
InChI
1S/C9H8O2/c10-8-5-6-11-9-4-2-1-3-7(8)9/h1-4H,5-6H2
MSTDXOZUKAQDRL-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
4-Chromanone has been used to study the substrate specificity and catalytic ability of 4-hydroxyacetophenone monooxygenase isolated from Pseudomonas fluorescens ACB.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
U Thull et al.
Biochemical pharmacology, 47(12), 2307-2310 (1994-06-15)
A number of unsubstituted aromatic hydrocarbons, azaheterocycles, oxaheterocycles and cyclic ketones were screened for their inhibitory potency towards monoamine oxidases (MAO; EC 1.4.3.4.) A and B. Fair activities (IC50 10-100 microM) and selectivities were found for, e.g. naphthalene, anthracene, phenanthrene
G R Harlow et al.
The Journal of biological chemistry, 272(9), 5396-5402 (1997-02-28)
Alanine-scanning mutagenesis was performed on amino acid residues 210-216 of cytochrome P450 3A4, the major drug-metabolizing enzyme of human liver. Mutagenesis of this region, which has been proposed to align with the C-terminal ends of F-helices from cytochromes P450BM-3, P450terp
Zahra Najafi et al.
Bioorganic chemistry, 83, 303-316 (2018-11-06)
A new series of tacrine-coumarin hybrids linked to 1,2,3-triazole were designed, synthesized, and tested as potent dual binding site cholinesterase inhibitors (ChEIs) for the treatment of Alzheimer's disease (AD). Among them, compound 8e was the most potent anti-AChE derivative (IC50 = 27 nM)
Gyeong-Je Lee et al.
Biological & pharmaceutical bulletin, 38(8), 1199-1207 (2015-08-04)
The aim of this study was to examine the anabolic and anticatabolic functions of bavachin in primary rat chondrocytes. With bavachin treatment, chondrocytes survived for 21 d without cell proliferation, and the proteoglycan content and extracellular matrix increased. Short-term monolayer culture
Angelika A Adamus-Grabicka et al.
Molecules (Basel, Switzerland), 23(12) (2018-12-06)
The aim of this study was to determine the cytotoxic effect of 3-arylidenechromanone (1) and 3arylideneflavanone (2) on HL-60 and NALM-6 cell lines (two human leukemia cell lines) and a WM-115 melanoma cell line. Both compounds exhibited high cytotoxic activity
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.