109835
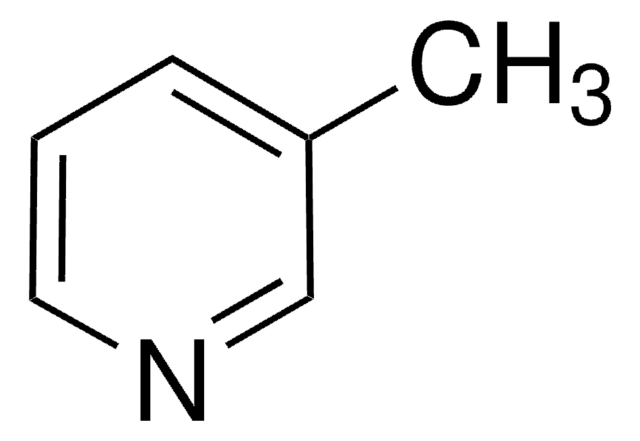
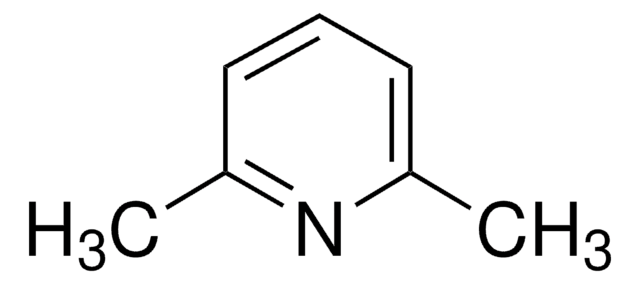
2-Methylpyridine
98%
Sinonimo/i:
2-Picoline, α-Picoline, 2-Methylpyridine, NSC 3409
About This Item
Prodotti consigliati
Densità del vapore
3.2 (vs air)
Livello qualitativo
Tensione di vapore
10 mmHg ( 24.4 °C)
Saggio
98%
Temp. autoaccensione
995 °F
Limite di esplosione
8.6 %
Indice di rifrazione
n20/D 1.500 (lit.)
P. eboll.
128-129 °C (lit.)
Punto di fusione
−70 °C (lit.)
Solubilità
H2O: freely soluble
alcohol: miscible
diethyl ether: miscible
Densità
0.943 g/mL at 25 °C (lit.)
Stringa SMILE
Cc1ccccn1
InChI
1S/C6H7N/c1-6-4-2-3-5-7-6/h2-5H,1H3
BSKHPKMHTQYZBB-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1C - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
84.2 °F - closed cup
Punto d’infiammabilità (°C)
29 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
US EPA Method 8270 (Appendix IX): GC Analysis of Semivolatiles on Equity®-5 (30 m x 0.25 mm I.D., 0.50 μm)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.