105449
γ-Thiobutyrolactone
98%
Sinonimo/i:
4-Butyrothiolactone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C4H6OS
Numero CAS:
Peso molecolare:
102.15
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Indice di rifrazione
n20/D 1.523 (lit.)
P. ebollizione
39-40 °C/1 mmHg (lit.)
Solubilità
THF: soluble
Densità
1.18 g/mL at 25 °C (lit.)
Gruppo funzionale
thioester
Stringa SMILE
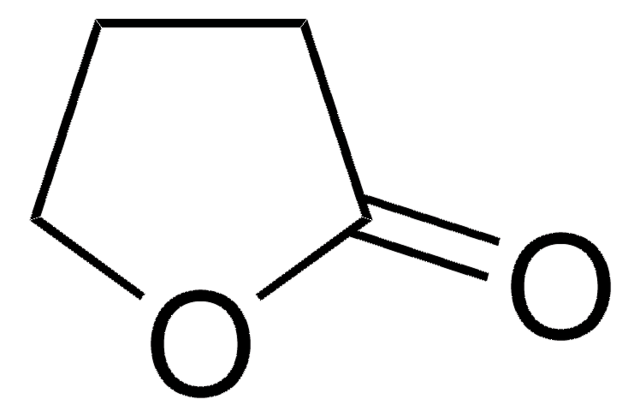
O=C1CCCS1
InChI
1S/C4H6OS/c5-4-2-1-3-6-4/h1-3H2
KMSNYNIWEORQDJ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
γ-Thiobutyrolactone undergoes copolymerization with glycidyl phenyl ether to form poly(ester-alt-sulfide).
Applicazioni
γ-Thiobutyrolactone was used to terminate the ring opening polymerization of ω-pentadecalactone to synthesize difunctional polyesters. γ-Thiobutyrolactone was used to study the mechanism of metabolism of sulphur containing heterocyclic compounds by lignin-degrading basidiomycete Coriolus versicolor.
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
186.8 °F - closed cup
Punto d’infiammabilità (°C)
86 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Tiny droplets make a big splash.
Michael Eisenstein
Nature methods, 3(2), 71-71 (2006-02-14)
Jonathan Garel et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(15), 4144-4152 (2006-02-03)
Homocysteine thiolactone (tHcy) is deemed a risk factor for cardiovascular diseases and strokes, presumably because it acylates the side chain of protein lysine residues ("N-homocysteinylation"), thereby causing protein damage and autoimmune responses. We analysed the kinetics of hydrolysis and aminolysis
Nishikubo et al.
Macromolecules, 31(15), 4746-4752 (1998-07-29)
Poly(ester-alt-sulfide) (polymer 1) was synthesized by the alternating copolymerization of glycidyl phenyl ether (GPE) with gamma-thiobutyrolactone (TBL) catalyzed by either quaternary onium salts or crown ether complexes. The copolymerization proceeded to produce polymer 1 with good yields in neat or
K D Holland et al.
Brain research, 615(1), 170-174 (1993-06-25)
Effects of alkyl-substituted gamma-butyrolactones and gamma-thiobutyrolactones on [35S]t-butylbicyclophosphorothionate (35S-TBPS) dissociation from the picrotoxinin receptor were studied. Unlike picrotoxinin, these lactones accelerated the dissociation rate of 35S-TBPS. Thus, previous reports that these lactones change the Kd but not the Bmax of
One-pot difunctionalization of poly (ω-pentadecalactone) with thiol-thiol or thiol-acrylate groups, catalyzed by Candida antarctica lipase B.
Takwa M, et al.
Macromolecular Rapid Communications, 27(22), 1932-1936 (2006)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.