179485
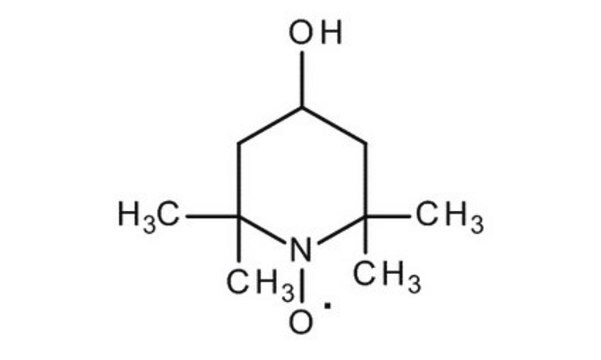
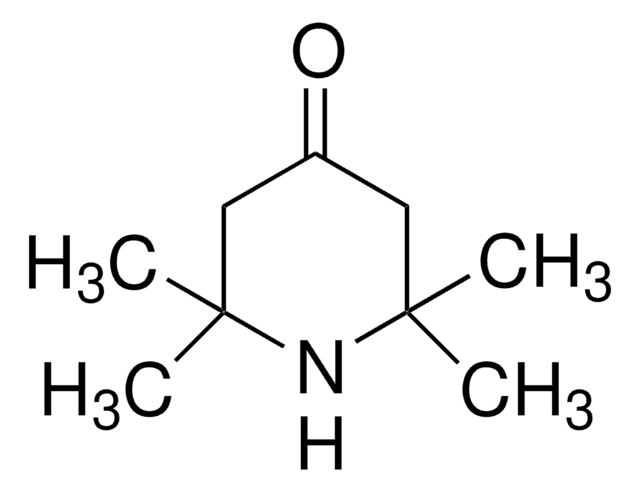
4-Oxo-TEMPO
Synonyme(s) :
4-Oxo-2,2,6,6-tetramethyl-1-piperidinyloxy, free radical
About This Item
Produits recommandés
Forme
solid
Niveau de qualité
Groupe fonctionnel
ketone
Température de stockage
2-8°C
Chaîne SMILES
CC1(C)CC(=O)CC(C)(C)N1[O]
InChI
1S/C9H16NO2/c1-8(2)5-7(11)6-9(3,4)10(8)12/h5-6H2,1-4H3
Clé InChI
WSGDRFHJFJRSFY-UHFFFAOYSA-N
Description générale
Application
- Redox sources for anodes in lithium secondary batteries
- Free-radical biological studies
- Radical spin-trapping
- Electron paramagnetic resonance studies
- Polymer chemisty and synthesis applications
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Acute 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique

![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)