357987
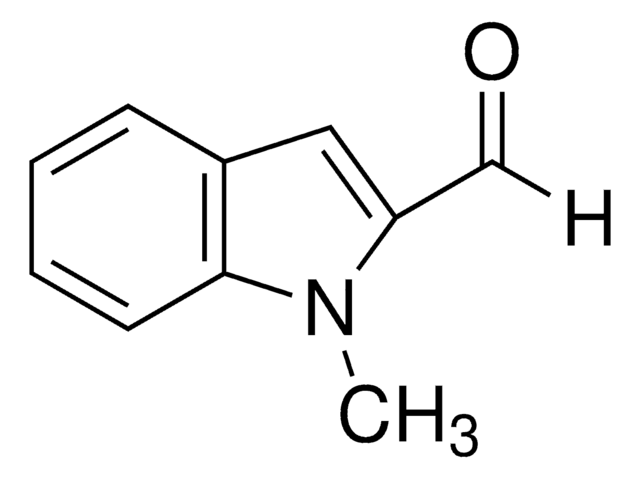
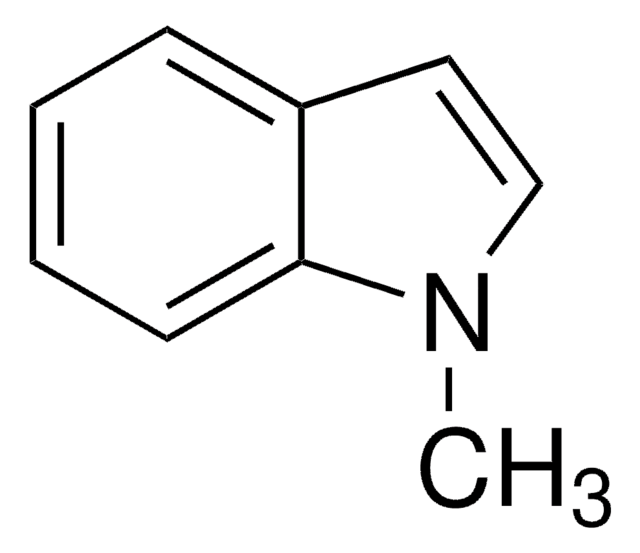
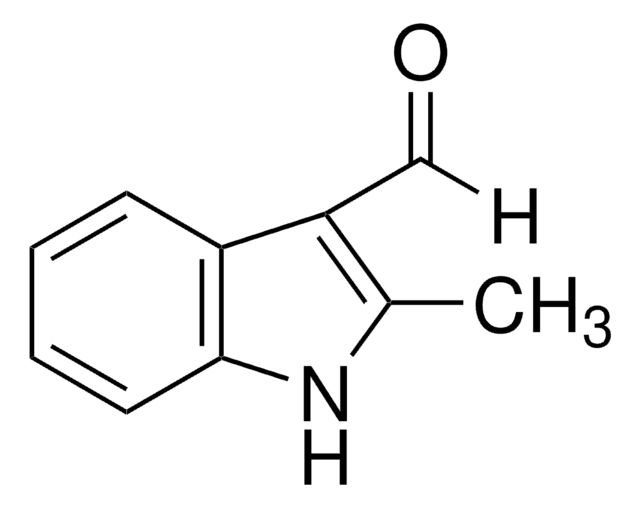
1-Methylindol-3-carboxaldehyd
97%
Synonym(e):
3-Formyl-1-methylindole, NSC 83042
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C10H9NO
CAS-Nummer:
Molekulargewicht:
159.18
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
mp (Schmelzpunkt)
70-72 °C (lit.)
SMILES String
Cn1cc(C=O)c2ccccc12
InChI
1S/C10H9NO/c1-11-6-8(7-12)9-4-2-3-5-10(9)11/h2-7H,1H3
InChIKey
KXYBYRKRRGSZCX-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung
1-Methylindole-3-carboxaldehyde is a heterocyclic indole aldehyde. 1-Methylindole-3-carboxaldehyde on condensation with 2-hydroxybenzohydrazide yields Schiff base.
Anwendung
1-Methylindole-3-carboxaldehyde may be used in the synthesis of (Z)-3-(1-methyl-1H-indol-3-yl)-2-(thiophen-3-yl)acrylonitrile, via base-catalyzed condensation with thiophene-3-acetonitrile. It was also used in the preparation of monomer, required for the synthesis of poly(3-vinyl-1-methylindole).
- Reactant for preparation of nitroolefins and β-nitroalcohols via microwave- or ultrasound-assisted Henry reactions
- Reactant for synthesis of quinolinones via three-component Ugi reaction
- Reactant for synthesis of α-ketoamides as inhibitors of Dengue virus protease with antiviral activity in cell-culture
- Reactant for preparation of thiazolopyrimidinones as inhibitors of Bcl-2 proteins
- Reactant for preparation of vinylindoles via Peterson olefination or olefination with Nysted reagent
- Reactant for preparation of indolyl alkenes from microwave-enhanced Knoevenagel condensation as antibacterial agents
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Vijayakumar N Sonar et al.
Acta crystallographica. Section C, Crystal structure communications, 60(Pt 3), o217-o218 (2004-03-09)
The title compound, C16H12N2S, has been synthesized by base-catalyzed condensation of 1-methylindole-3-carboxaldehyde with thiophene-3-acetonitrile. The product assumes an approximately planar Z configuration. The molecule has a thienyl-ring flip disorder.
Preliminary analysis of the 1H-and 13C-NMR spectra of poly (3-vinyl-1-methylindole).
Trumbo DL.
Polymer Bull., 37(1), 75-80 (1996)
Wagee A Yehye et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 9), o1824-o1824 (2008-01-01)
In the crystal structure of the title Schiff-base, C(20)H(21)N(3)O(4), the amino group forms an N-H⋯O hydrogen bond to the acetyl group of an adjacent mol-ecule, forming a zigzag chain. The 2-hydr-oxy group is inter-nally hydrogen bonded to the amido group
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.