Alle Fotos(2)
Wichtige Dokumente
262439
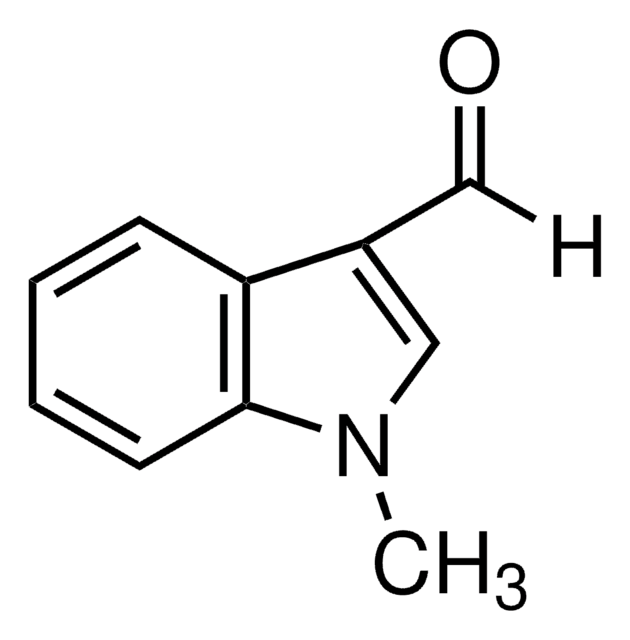
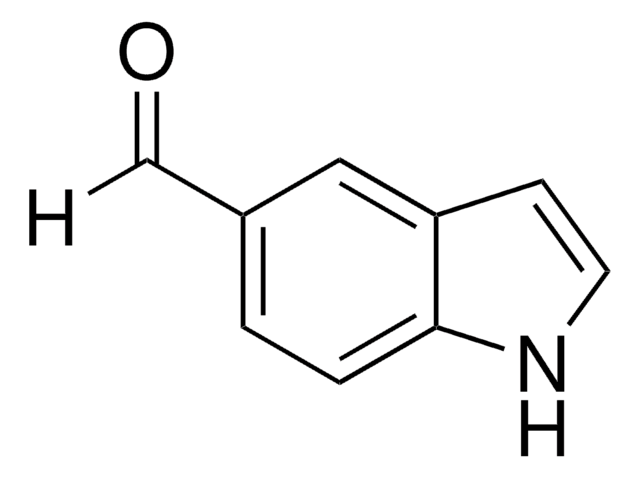
2-Methylindol-3-carboxaldehyd
97%
Synonym(e):
3-Formyl-2-methylindole, NSC 11895
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C10H9NO
CAS-Nummer:
Molekulargewicht:
159.18
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
mp (Schmelzpunkt)
200-201 °C (lit.)
Funktionelle Gruppe
aldehyde
SMILES String
Cc1[nH]c2ccccc2c1C=O
InChI
1S/C10H9NO/c1-7-9(6-12)8-4-2-3-5-10(8)11-7/h2-6,11H,1H3
InChIKey
CYZIVXOEJNAIBS-UHFFFAOYSA-N
Allgemeine Beschreibung
Oxidative activation of 2-methylindole-3-carboxaldehyde via N-heterocyclic carbene organocatalysis generates heterocyclic ortho-quinodimethane as a key intermediate.
Anwendung
2-Methylindole-3-carboxaldehyde has been used in the preparation of 1-phenylsulfonyl-2-methylindole-3-carboxaldehyde.
Reactant for preparation of:
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Fluorescent sensors (BODIPY)
- Antimicrobial agents against methicillin-resistant Staphylococcus aureus
- G protein-coupled receptor CRTh2 antagonists
- Inhibitors of PI3 kinase-α
- Antitubercular agents
- Anti-inflammatory agents
- Mycobacterium tuberculosis protein tyrosine phosphatase B
- Glucocorticoid receptor ligands
- Agents stimulating neurite outgrowth
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Xingkuan Chen et al.
Angewandte Chemie (International ed. in English), 52(42), 11134-11137 (2013-09-17)
Aryl aldehyde activation: Oxidative activation of 2-methylindole-3-carboxaldehyde (I) through N-heterocyclic carbene (NHC) organocatalysis generates heterocyclic ortho-quinodimethane (II) as a key intermediate. This intermediate then undergoes formal [4+2] cycloaddition with trifluoromethyl ketones or isatins to form polycyclic lactones containing a quaternary
G Chakkaravarthi et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 2), o542-o542 (2008-01-01)
In the title compound, C(16)H(15)NO(3)S, the plane of the phenyl ring forms a dihedral angle of 80.37 (8)° with the indole ring system. The crystal packing is stabilized by weak O-H⋯O hydrogen bonds which link the mol-ecules into infinite chains along
Ming-Zhi Zhang et al.
European journal of medicinal chemistry, 92, 776-783 (2015-01-31)
Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.