1222818
USP
Dissolution Performance Verification Standard - Prednisone (30 tablets)
United States Pharmacopeia (USP) Reference Standard
Sinônimo(s):
DPVS-Prednisone
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Código UNSPSC:
41116107
NACRES:
NA.24
Produtos recomendados
grau
pharmaceutical primary standard
Agency
certified by the USP
família API
prednisone
Formulário
tablet
fabricante/nome comercial
USP
concentração
10 mg/tablet (nominal prednisone content per tablet)
aplicação(ões)
pharmaceutical
Formato
mixture
temperatura de armazenamento
room temp
Descrição geral
The USP Performance Verification Test (PVT) is an essential aspect of the General Chapter <711> Dissolution that assesses the comprehensive performance of Dissolution Apparatus 1 (basket) and Dissolution Apparatus 2 (paddle). DPVS - Prednisone Tablets is specially engineered by USP to use as a reference standard for PVT of apparatus used in dissolution testing for the following reasons:
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, has been developed and issued under the authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
- High stability and ease of use
- High sensitivity to operational and mechanical variables of instrument setup
- Low sensitivity to media degassing
- Low tablet-to-tablet variability
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, has been developed and issued under the authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Aplicação
Dissolution Performance Verification Standard (DPVS) – Prednisone tablets USP reference standard is used for the comprehensive qualification of dissolution instruments using performance verification testing (PVT) to achieve reliable results that reflect the quality of the drug product.
Componentes
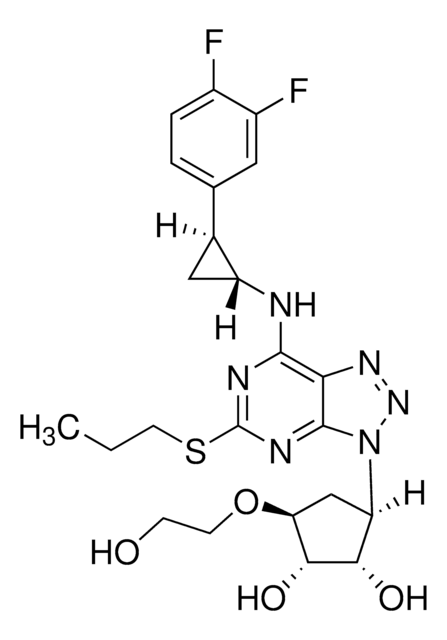
Each tablet contains: Dicalcium Phosphate Anhydrous (7757-93-9), Microcrystalline Cellulose (9004-34-6), Colloidal silicone dioxide (112945-52-5), Magnesium Stearate (557-04-0), Prednisone (53-03-2), Sodium Dodecyl Sulfate Fine (151-21-3), Sodium Starch Glycolate Type A (9063-38-1)
Nota de análise
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
Outras notas
Sales restrictions may apply.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
STOT RE 2 Inhalation
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica