442232-U
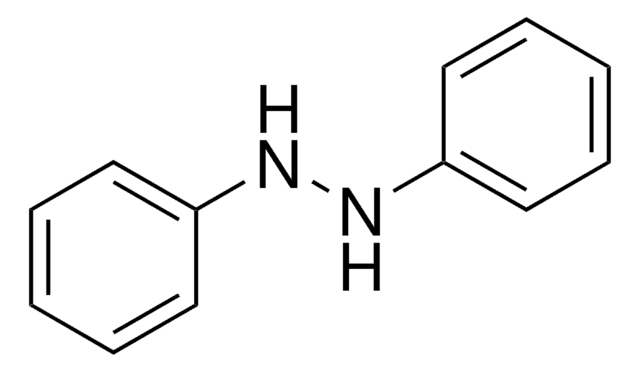
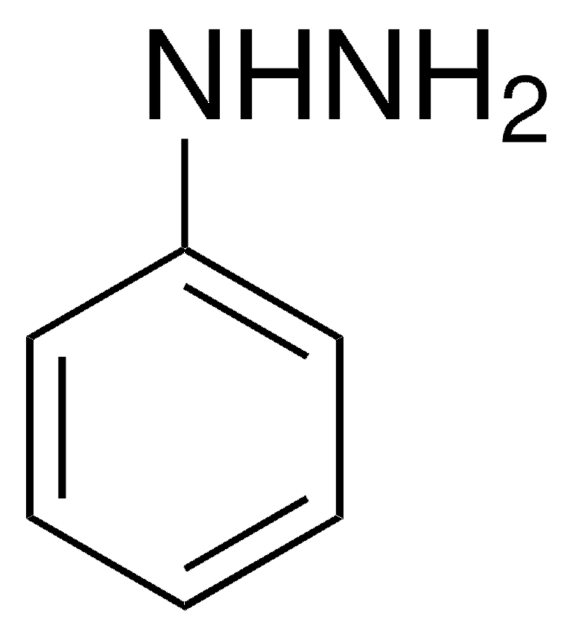
1,2-Diphenylhydrazine

analytical standard, ampule of 100 mg
Sinônimo(s):
Hydrazobenzene, N,N′-Diphenylhydrazine, N,N′-Bianiline, NSC 3510
About This Item
Produtos recomendados
grau
analytical standard
Certificado de análise (CofA)
current certificate can be downloaded
embalagem
ampule of 100 mg
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
pf
123-126 °C (lit.)
aplicação(ões)
cleaning products
cosmetics
environmental
food and beverages
personal care
Formato
neat
temperatura de armazenamento
2-30°C
cadeia de caracteres SMILES
N(Nc1ccccc1)c2ccccc2
InChI
1S/C12H12N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10,13-14H
chave InChI
YBQZXXMEJHZYMB-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- Insertion reactions with organometallic tantalum complexes
- Reduction reactions catalyzed by titanium(III) trichloride yielding amines
- Studying the mechanism of hydrazobenzene rearrangement
- Reaction with N-heterocyclic stable silylene
- Synthesis of dimanganese amide hydrazide cluster complexes
- Iron-mediated hydrazine reductions yielding iron arylimide cubanes
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B
Código de classe de armazenamento
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves, type P2 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica