T3787
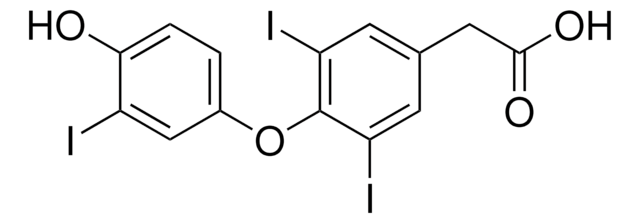
3,3′,5,5′-Tetraiodothyroacetic acid
≥98% (TLC), powder, thyrointegrin receptor antagonist
Sinônimo(s):
4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodobenzeneacetic acid, Tetrac
About This Item
Produtos recomendados
Nome do produto
3,3′,5,5′-Tetraiodothyroacetic acid,
solubilidade
acetone: soluble 19.60-20.40 mg/mL, clear, colorless (or faintly yellow to yellow)
Nível de qualidade
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
OC(=O)Cc1cc(I)c(Oc2cc(I)c(O)c(I)c2)c(I)c1
InChI
1S/C14H8I4O4/c15-8-4-7(5-9(16)13(8)21)22-14-10(17)1-6(2-11(14)18)3-12(19)20/h1-2,4-5,21H,3H2,(H,19,20)
chave InChI
PPJYSSNKSXAVDB-UHFFFAOYSA-N
Categorias relacionadas
Aplicação
- as a positive control to study the effects of ioxynil (IOX)
- diethylstilbestrol (DES) exposure on zebrafish embryos
- to study its effects on long-term potentiation (LTP) and long-term depression (LTD) in the dentate gyrus in urethane-anesthetized male rats
- to determine its influence on the actions of thyroid-stimulating hormone
- thyroid-stimulating immunoglobulins in orbital fibroblast
Ações bioquímicas/fisiológicas
Ligação
Nota de preparo
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 2 Oral
Código de classe de armazenamento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica