T1143
Trypsinogen from bovine pancreas
essentially salt-free, lyophilized powder, ≥10,000 BAEE units/mg protein (E1%/280, after activation to trypsin)
About This Item
Produtos recomendados
fonte biológica
bovine pancreas
Nível de qualidade
Ensaio
85-100% (UV)
Formulário
essentially salt-free, lyophilized powder
atividade específica
≥10,000 BAEE units/mg protein (E1%/280, after activation to trypsin)
peso molecular
23,981 Da by calculation
técnica(s)
mass spectrometry (MS): suitable
solubilidade
H2O: soluble 10 mg/mL
nº de adesão UniProt
temperatura de armazenamento
−20°C
Informações sobre genes
bovine ... TRYP8(282603)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- the secondary structure analysis of proteins in H2O solution using single-pass attenuated total reflection Fourier transform infrared (ATR-FT-IR) microscopy
- tuning and calibration of electrospray ionization quadrupole time-of-flight (ESI-Q-TOF) mass spectrometer
- the secondary structure analysis of proteins by infrared (IR) spectroscopy
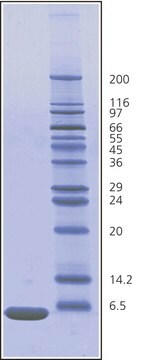
- SDS-PAGE as a molecular weight standard (24kDa)
Ações bioquímicas/fisiológicas
Definição da unidade
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Protocolos
Enzymatic Assay of Urease from Jack Beans
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica