SML2521
BRD6929
≥98% (HPLC)
Sinônimo(s):
4-(Acetylamino)-N-[2-amino-5-(2-thienyl)phenyl]benzamide, BRD6929, Compound 60, TPB
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C19H17N3O2S
Número CAS:
Peso molecular:
351.42
Número MDL:
Código UNSPSC:
12352200
NACRES:
NA.77
Produtos recomendados
Ensaio
≥98% (HPLC)
forma
powder
cor
white to beige
solubilidade
DMSO: 2 mg/mL, clear
temperatura de armazenamento
−20°C
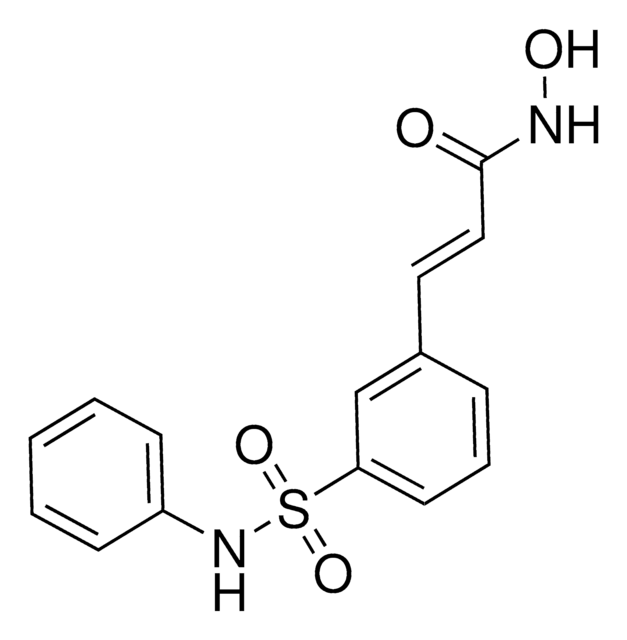
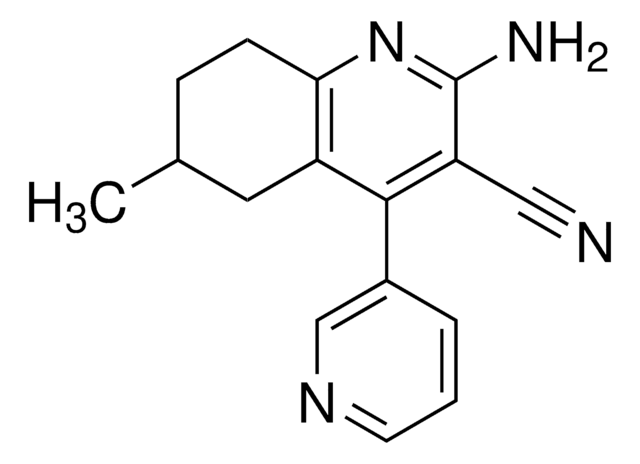
cadeia de caracteres SMILES
CC(NC1=CC=C(C(NC2=C(N)C=CC(C3=CC=CS3)=C2)=O)C=C1)=O
Ações bioquímicas/fisiológicas
TPB is a potent and selective inhibitor of HDAC1 and HDAC2. TPB potentiates gnidimacrin activation of latent HIV-1 in cells.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Li Huang et al.
ACS medicinal chemistry letters, 9(3), 268-273 (2018-03-16)
We have previously reported gnidimacrin (GM), a protein kinase C (PKC) agonist, significantly reduces the frequency of HIV-1 latently infected cells in peripheral blood mononuclear cells (PBMCs) from patients undergoing successful antiretroviral therapy at low picomolar concentrations ex vivo, which
Stefan Kubicek et al.
Proceedings of the National Academy of Sciences of the United States of America, 109(14), 5364-5369 (2012-03-22)
Under the instruction of cell-fate-determining, DNA-binding transcription factors, chromatin-modifying enzymes mediate and maintain cell states throughout development in multicellular organisms. Currently, small molecules modulating the activity of several classes of chromatin-modifying enzymes are available, including clinically approved histone deacetylase (HDAC)
F F Wagner et al.
Chemical science, 6(1), 804-815 (2015-02-03)
Aiming towards the development of novel nootropic therapeutics to address the cognitive impairment common to a range of brain disorders, we set out to develop highly selective small molecule inhibitors of HDAC2, a chromatin modifying histone deacetylase implicated in memory
Joey L Methot et al.
Bioorganic & medicinal chemistry letters, 18(3), 973-978 (2008-01-10)
We report herein the initial exploration of novel selective HDAC1/HDAC2 inhibitors (SHI-1:2). Optimized SHI-1:2 structures exhibit enhanced intrinsic activity against HDAC1 and HDAC2, and are greater than 100-fold selective versus other HDACs, including HDAC3. Based on the SAR of these
Andrew J Wilson et al.
Cancer biology & therapy, 12(6), 484-493 (2011-07-09)
High grade epithelial ovarian cancers are relatively sensitive to DNA damaging platinum-based chemotherapy, suggesting that the dependencies of ovarian tumors on DNA damage response pathways can be harnessed for therapeutic purposes. Our goal was to determine if the DNA damage
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica