M9411
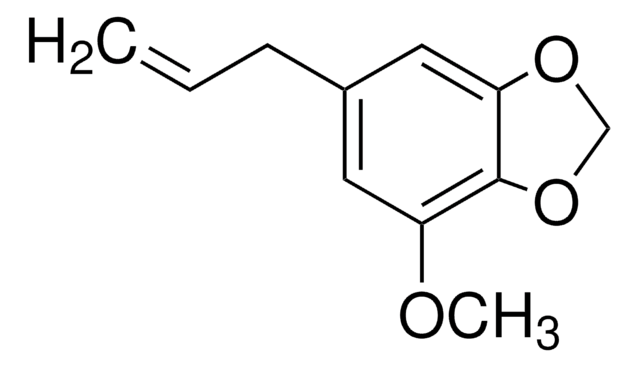
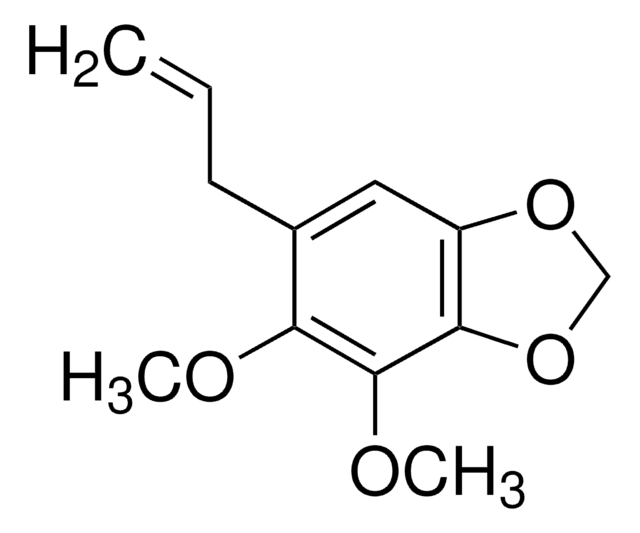
Myristicin from parsley leaf oil
≥85% (HPLC), oil
Sinônimo(s):
4-Methoxy-6-(2-propenyl)-1,3-benzodioxole
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C11H12O3
Número CAS:
Peso molecular:
192.21
Número CE:
Número MDL:
Código UNSPSC:
12352205
ID de substância PubChem:
NACRES:
NA.77
Produtos recomendados
Nível de qualidade
Ensaio
≥85% (HPLC)
Formulário
oil
cor
clear light yellow
aplicação(ões)
metabolomics
vitamins, nutraceuticals, and natural products
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
COc1cc(CC=C)cc2OCOc12
InChI
1S/C11H12O3/c1-3-4-8-5-9(12-2)11-10(6-8)13-7-14-11/h3,5-6H,1,4,7H2,2H3
chave InChI
BNWJOHGLIBDBOB-UHFFFAOYSA-N
Descrição geral
Myristicin, a phenylpropene, is an essential oil component. It has anticholinergic and psychotropic activities. Myristicin blocks cytochrome P450 monooxygenases, which detoxifies furanocoumarins. It functions as a serotonin receptor agonist and hallucinogenic agent. Myristicin stimulates glutathione S-transferase activity and might function as a chemopreventive agent. It acts as a precursor for the metabolite 3,4- methylenedioxymethamphetamine (MDMA).
Aplicação
Myristicin from parsley leaf oil has been used to study competitive and uncompetitive inhibition of ACP (acid phosphatase) and ALP (alkaline phosphatase).
Ações bioquímicas/fisiológicas
Myristicin induces the expression of glutathione S-transferase and cytochrome P450 (Cyp1a-1) in liver cells. May enhance detoxification of carcinogenic substances.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
STOT SE 3
Órgãos-alvo
Central nervous system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
H S Lee et al.
Journal of chromatography. B, Biomedical sciences and applications, 705(2), 367-372 (1998-04-01)
Myristicin [5-allyl-1-methoxy-2,3-(methylenedioxy)benzene] is a flavoring plant constituent and has been known to produce significant psychopharmacological responses as well as insecticidal activity. From in vitro and in vivo metabolism of myristicin, the two metabolites 5-allyl-1-methoxy-2,3-dihydroxybenzene and 1'-hydroxymyristicin were identified using GC-MS
G Q Zheng et al.
Carcinogenesis, 13(10), 1921-1923 (1992-10-01)
Glutathione S-transferase (GST) assay-guided fractionation of parsley leaf oil from the edible plant Petroselinum sativum Hoffm. (Umbelliferae) led to the isolation of myristicin. Myristicin showed high activity as an inducer of the detoxifying enzyme GST in the liver and small
H Ahmad et al.
Biochemical and biophysical research communications, 236(3), 825-828 (1997-07-30)
The present studies were undertaken to elucidate the mechanism of induction of glutathione S-transferase (GST) in mouse liver by myristicin, an active constituent of parsley leaf. A/J albino mice, given 5 to 50 mg doses of myristicin, showed 4- to
Allelochemical induction of cytochrome P450 monooxygenases and amelioration of xenobiotic toxicity in Helicoverpa zea
Zeng RS, et al.
Journal of Chemical Ecology, 33(3), 449-449 (2007)
Myristicin-induced neurotoxicity in human neuroblastoma SK-N-SH cells
Lee BK, et al.
Toxicology Letters, 157(1), 49-56 (2005)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica