C5614
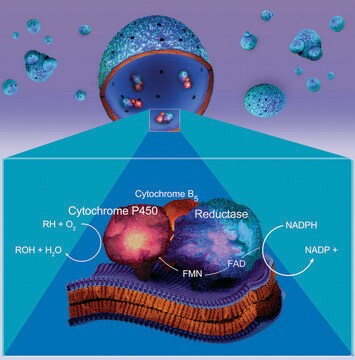
Cytochrome P450 human
1A2 Isozyme Microsomes, with P450 Reductase and cytochrome b5, recombinant, expressed in baculovirus infected insect cells (BTI-TN-5B1-4)
About This Item
Produtos recomendados
fonte biológica
human
Nível de qualidade
recombinante
expressed in baculovirus infected insect cells (BTI-TN-5B1-4)
forma
solution
peso molecular
58 kDa
embalagem
vial of 0.5 nmol
nº de adesão UniProt
aplicação(ões)
cell analysis
Condições de expedição
dry ice
temperatura de armazenamento
−70°C
Informações sobre genes
human ... CYP1A2(1544)
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Ações bioquímicas/fisiológicas
Outras notas
forma física
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica