A6888
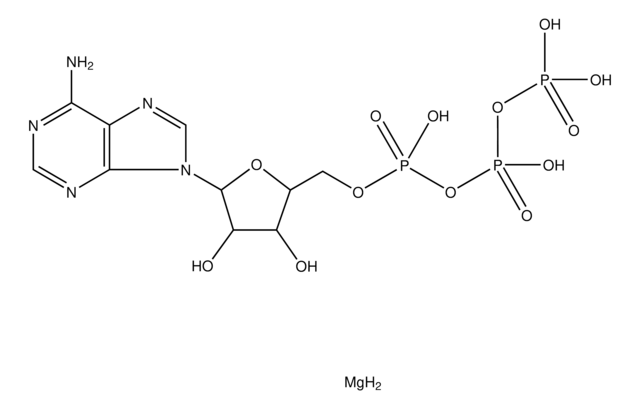
Adenosine 5′-triphosphate–Agarose
aqueous glycerol suspension
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Produtos recomendados
forma
aqueous glycerol suspension
Nível de qualidade
Extensão da rotulagem
≥1 μmol per mL
matriz
cross-linked 4% beaded agarose
ativação da matriz
cyanogen bromide
ligação da matriz
ribose hydroxyls
espaçador de matriz
11 atoms (adipic acid dihydrazide)
temperatura de armazenamento
−20°C
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Adenosine 5′-triphosphate Agarose (5′-ATP agarose) has been used in affinity chromatography to purify uridine kinase from Ehrlich ascites tumor cells.
forma física
Suspension in 50% glycerol containing 0.25 M NaCl
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
B L Stitt
The Journal of biological chemistry, 263(23), 11130-11137 (1988-08-15)
We have determined that 3 mol of ATP or other adenine nucleotide can bind to Escherichia coli transcription termination protein rho, in the presence or absence of the RNA cofactor that is required for activation of rho's ATPase activity. Isotope
S Ogg et al.
The Journal of biological chemistry, 269(48), 30461-30469 (1994-12-02)
Human Cdc25C is a protein phosphatase that dephosphorylates and activates Cdc2-cyclin B to trigger entry into mitosis. Cdc25C is itself regulated by phosphorylation. In asynchronously growing HeLa cells, we have determined that serine 216 is the major site of Cdc25C
V Nagaraja et al.
Journal of molecular biology, 182(4), 579-587 (1985-04-20)
We have purified the type I restriction enzymes SB and SP from Salmonella typhimurium and S. potsdam, respectively, and determined the DNA sequences that they recognize. These sequences resemble those previously determined for the type I enzymes, EcoB, EcoK and
H D Kim et al.
Biochemistry, 38(44), 14697-14710 (1999-11-05)
Two polynucleotide-dependent ATPases, 95 and 181 kDa in size, have been purified to near homogeneity from cell-free extracts of Schizosaccharomyces pombe. Despite their size differences, their biochemical properties were strikingly similar. Both enzymes were capable of unwinding RNA and DNA
B Suri et al.
The EMBO journal, 3(3), 575-579 (1984-03-01)
The EcoA restriction enzyme from Escherichia coli 15T- has been isolated. It proves to be an unusual enzyme, clearly related functionally to the classical type I restriction enzymes. The basic enzyme is a two subunit modification methylase. Another protein species
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica