41719
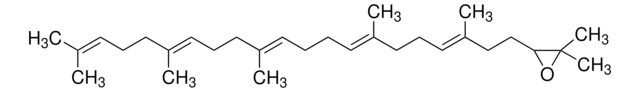
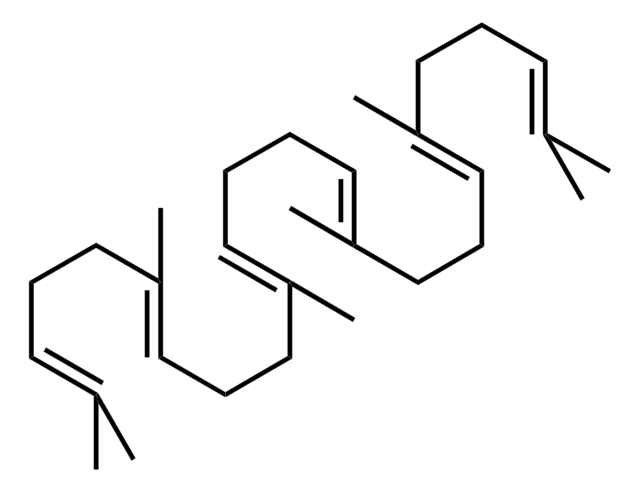
(3S)-2,3-Oxidosqualene
≥97.5% (HPLC)
Sinônimo(s):
(S)-2,3-Epoxy-2,3-dihydrosqualene, (S)-22,23-Epoxy-2,6,10,15,19,23-hexamethyl-2,6,10,14,18-tetracosapentaene, (S)-Squalene 2,3-epoxide, (S)-Squalene 2,3-oxide
About This Item
Produtos recomendados
Ensaio
≥97.5% (HPLC)
Formulário
liquid
pureza óptica
enantiomeric excess: ≥90.0%
adequação
conforms to structure for Proton NMR spectrum
temperatura de armazenamento
−20°C
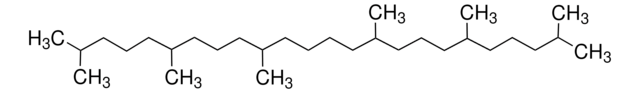
cadeia de caracteres SMILES
CC1(C)O[C@H]1CC/C(C)=C/CC/C(C)=C/CC/C=C(C)/CC/C=C(C)/CCC=C(C)C
InChI
1S/C30H50O/c1-24(2)14-11-17-27(5)20-12-18-25(3)15-9-10-16-26(4)19-13-21-28(6)22-23-29-30(7,8)31-29/h14-16,20-21,29H,9-13,17-19,22-23H2,1-8H3/b25-15+,26-16+,27-20+,28-21+/t29-/m0/s1
chave InChI
QYIMSPSDBYKPPY-RSKUXYSASA-N
Categorias relacionadas
Aplicação
- Schizophrenic behavior of 2,3-Oxidosqualene Sterol Cyclase from pig liver towards 2,3-oxidosqualene analogues. Alain Krief and colleagues discuss the variable catalytic behaviors of oxidosqualene cyclase when interacting with different oxidosqualene analogues, suggesting implications for sterol production efficiency and specificity (Krief et al., 2021).
Ações bioquímicas/fisiológicas
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica