238422
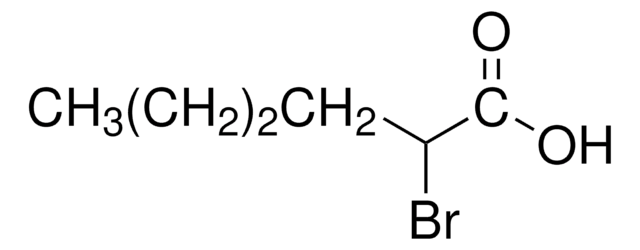
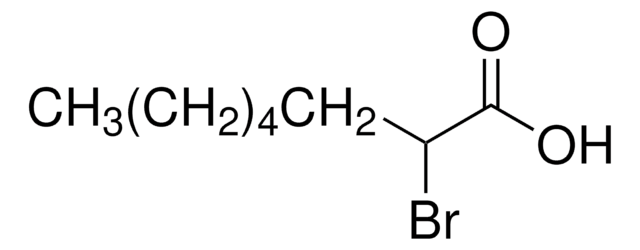
2-Bromohexadecanoic acid
~97%
Sinônimo(s):
2-Bromopalmitic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH3(CH2)13CH(Br)CO2H
Número CAS:
Peso molecular:
335.32
Beilstein:
1726517
Número CE:
Número MDL:
Código UNSPSC:
12352203
ID de substância PubChem:
NACRES:
NA.77
Produtos recomendados
Ensaio
~97%
Nível de qualidade
Formulário
solid
cadeia de caracteres SMILES
CCCCCCCCCCCCCCC(Br)C(O)=O
InChI
1S/C16H31BrO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15(17)16(18)19/h15H,2-14H2,1H3,(H,18,19)
chave InChI
DPRAYRYQQAXQPE-UHFFFAOYSA-N
Informações sobre genes
human ... PPARD(5467)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
PPARδ (peroxisome proliferator-activated receptor, delta isoform) acts as a transcription factor for gene expression as well as playing a role in lipid metabolism regulation; activity of this receptor is ligand-regulated. 2-Bromohexadecanoic acid is a metabolically stable analoge of the fatty acid palmitic acid that has been shown to be a natural ligand for the PPARδ receptor. 2-Bromohexadecanoic acid has also been used in studies of fatty acid oxidation, palmitoylation, and glucose uptake.
Ações bioquímicas/fisiológicas
2-Bromohexadecanoic acid is a PPARδ agonist. It has also been shown to inhibit fatty acid oxidation, inhibit DHHC-mediated palmitoylation, and promote glucose uptake in rat cardiac cells and the insulin-sensitive murine fibroblast line A31-IS.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Joel Berger et al.
Annual review of medicine, 53, 409-435 (2002-01-31)
The peroxisome proliferator-activated receptors (PPARs) are a group of three nuclear receptor isoforms, PPAR gamma, PPAR alpha, and PPAR delta, encoded by different genes. PPARs are ligand-regulated transcription factors that control gene expression by binding to specific response elements (PPREs)
Abiy M Mohammed et al.
Biochemical pharmacology, 85(1), 109-114 (2012-10-25)
Phagocyte-like NADPH oxidase (Nox2) has been shown to play regulatory roles in the metabolic dysfunction of the islet β-cell under the duress of glucolipotoxic conditions and exposure to proinflammatory cytokines. However, the precise mechanisms underlying Nox2 activation by these stimuli
Aaron C Baldwin et al.
American journal of physiology. Endocrinology and metabolism, 302(11), E1390-E1398 (2012-03-23)
Exposure of insulin-producing cells to elevated levels of the free fatty acid (FFA) palmitate results in the loss of β-cell function and induction of apoptosis. The induction of endoplasmic reticulum (ER) stress is one mechanism proposed to be responsible for
Kenji Hayata et al.
Journal of pharmacological sciences, 108(3), 348-354 (2008-11-15)
To obtain compounds that promote glucose uptake in muscle cells, the novel cell lines A31-IS derived from Balb/c 3T3 A31 and C2C12-IS from mouse myoblast C2C12 were established. In both cell lines, glucose consumption was induced by insulin and suppressed
Benjamin C Jennings et al.
Journal of lipid research, 50(2), 233-242 (2008-10-02)
Pharmacologic approaches to studying palmitoylation are limited by the lack of specific inhibitors. Recently, screens have revealed five chemical classes of small molecules that inhibit cellular processes associated with palmitoylation (Ducker, C. E., L. K. Griffel, R. A. Smith, S.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica