04685
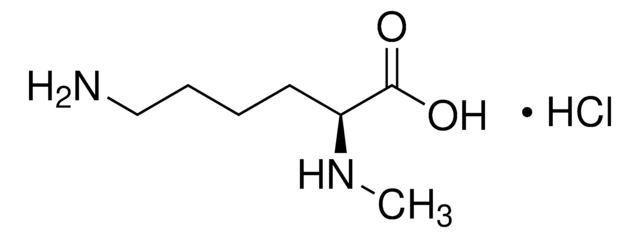
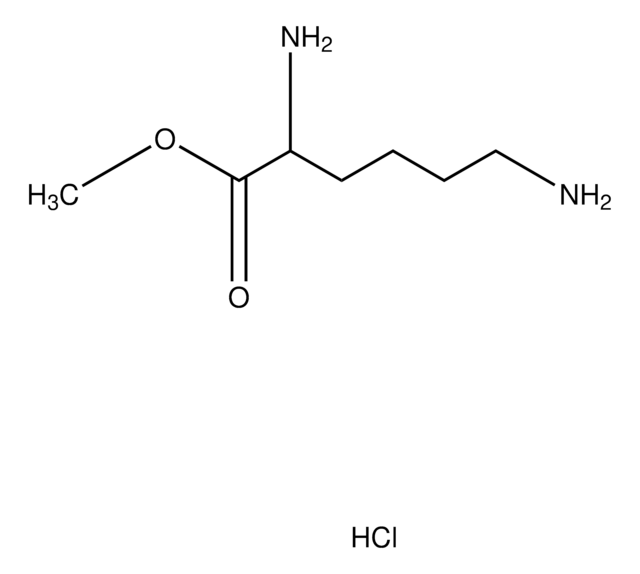
Nε-Methyl-L-lysine hydrochloride
≥98.0% (TLC)
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C7H16N2O2 · HCl
Número CAS:
Peso molecular:
196.68
Número CE:
Número MDL:
Código UNSPSC:
12352202
ID de substância PubChem:
NACRES:
NA.32
Produtos recomendados
Nível de qualidade
Ensaio
≥98.0% (TLC)
atividade óptica
[α]/D 20.5±1.5°, c = 0.1 in 1 M HCl
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
Cl.CNCCCC[C@H](N)C(O)=O
Cl.CNCCCC[C@H](N)C(O)=O
InChI
1S/C7H16N2O2.ClH/c1-9-5-3-2-4-6(8)7(10)11;/h6,9H,2-5,8H2,1H3,(H,10,11);1H/t6-;/m0./s1
chave InChI
AQELUQTVJOFFBN-RGMNGODLSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Ações bioquímicas/fisiológicas
N ε-methyl-L-lysine was identified as a lysine analog with inhibitory effects on the growth and sporulation of Penicillium chrysogenum and benzyl-penicillin formation by mycelia.
Embalagem
Bottomless glass bottle. Contents are inside inserted fused cone.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
F Zappacosta et al.
European journal of biochemistry, 222(3), 761-767 (1994-06-15)
Advanced mass spectrometric procedures have been extensively used to provide an accurate structural characterization of aspartate aminotransferase from Sulfolobus solfataricus. The amino acid sequence of this enzyme had previously been deduced from the DNA sequence. The accurate molecular mass of
H Kalász et al.
Journal of chromatographic science, 43(4), 165-168 (2005-06-25)
Administration of (14)C-labelled L-deprenyl to rats results in the urinary elimination of a 14C-labelled compound. The 9-fluorenylmethoxycarbonyl chloride-reacted urine sample is fractionated by high-performance liquid chromatography (HPLC) on an octadecyl silica stationary phase. N(epsilon)-Monomethyl-lysine is identified in the fraction containing
M Moracci et al.
Enzyme and microbial technology, 17(11), 992-997 (1995-11-01)
The gene coding for the beta-glycosidase from the archaeon Sulfolobus solfataricus has been overexpressed in Escherichia coli. The enzyme was purified to homogeneity with a rapid purification procedure employing a thermal precipitation as a crucial step. The final yield was
H Kalász et al.
Journal of chromatography. A, 1079(1-2), 208-212 (2005-07-26)
Nepsilon-Monomethyllysine was identified in the serum, urine, brain, and liver samples of rats treated per os with L-deprenyl. The identification procedure included reaction with Fmoc chloride, clean-up, and analysis using HPLC-UV-MS. Oral administration of (-)-N-14C-methyl-N-propynyl(2-phenyl-1-methyl)ethylammonium hydrochloride L-deprenyl) to rats resulted
M Kushiro et al.
Nephron, 79(4), 458-468 (1998-08-05)
Increases in extracellular matrix (ECM) and changes in its components have been documented in the glomeruli of diabetic nephropathy. Advanced glycation end products formed by glycoxidation have been shown to induce the synthesis of ECM components and transforming growth factor
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica