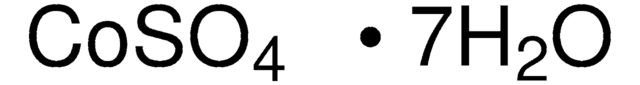C6768
Cobalt(II) sulfate heptahydrate
ReagentPlus®, ≥99%
Sinônimo(s):
Cobaltous sulfate heptahydrate
About This Item
Produtos recomendados
Nível de qualidade
linha de produto
ReagentPlus®
Ensaio
≥99%
forma
powder
pH
4 (20 °C, 100 g/L)
densidade
2.03 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
O.[Co++].[O-]S([O-])(=O)=O
InChI
1S/Co.H2O4S.H2O/c;1-5(2,3)4;/h;(H2,1,2,3,4);1H2/q+2;;/p-2
chave InChI
BGORGFZEVHFAQU-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- Benzazoles from alcohols and o-substituted anilines.
- β-Acetamido ketones via one-pot multi-component coupling reaction of aromatic aldehydes, acetophenones, and acetyl chlorides.
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Irrit. 2 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Código de classe de armazenamento
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Dr. Schmuch, Dr. Siozios, Professor Dr. Winter, and Dr. Placke review the challenges and opportunities of nickelrich layered oxide cathode materials. They discuss production processes for the layered oxide cathode materials as well as their chemistry and morphology.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica