64643
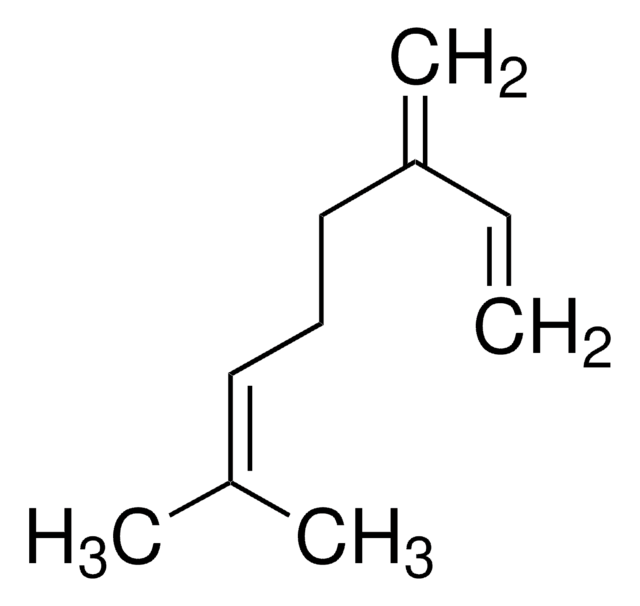
Mirceno
analytical standard
Sinônimo(s):
ββ-Mirceno, 7-Metil-3-metileno-1,6-octadieno
About This Item
Produtos recomendados
grau
analytical standard
Nível de qualidade
densidade de vapor
4.7 (vs air)
pressão de vapor
~7 mmHg ( 20 °C)
Ensaio
≥90.0% (GC)
prazo de validade
limited shelf life, expiry date on the label
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
índice de refração
n20/D 1.469 (lit.)
p.e.
167 °C (lit.)
densidade
0.791 g/mL at 25 °C (lit.)
aplicação(ões)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
Formato
neat
temperatura de armazenamento
−20°C
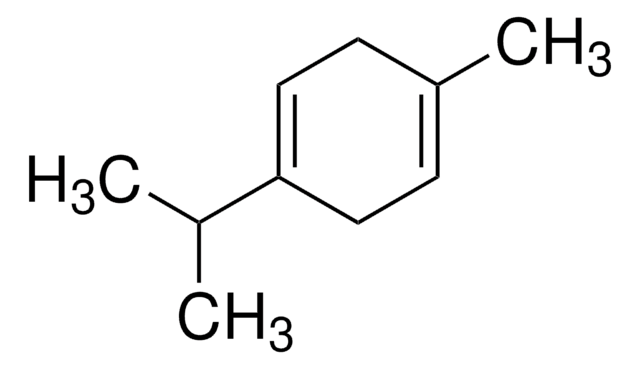
cadeia de caracteres SMILES
C\C(C)=C/CCC(=C)C=C
InChI
1S/C10H16/c1-5-10(4)8-6-7-9(2)3/h5,7H,1,4,6,8H2,2-3H3
chave InChI
UAHWPYUMFXYFJY-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Aplicação
- Determination of four flavor compounds— isoamyl acetate, ethyl hexanoate, benzaldehyde, and myrcene, in commercial beer samples by using two solvent-less sample treatment techniques of stir-bar sorptive extraction (SBSE) and solid-phase microextraction (SPME) for their subsequent analysis by gas chromatography-flame ionization detection (GC-FID)
- Analysis of fresh mano samples for the detection and quantification of myrcene using a quartz crystal microbalance (QCM) sensor, modified with ethyl cellulose
- Multi-residue analysis of volatiles and fatty acids found in wild and cultivated fennel samples by a single extraction method and gas chromatographic-flame ionization detection (GC-FID)
- Identification and determination of volatile aroma compounds, commonly present in three plant species from the Citrus genus by using simultaneous distillation extraction (SDE) technique for sample treatment and analysis by gas chromatography-mass spectrometry (GC-MS) in electron ionization mode (EI)
- Secondary metabolite profiling of various plant parts collected from 82 plants belonging to 21 different cannabis strains using gas chromatography-mass spectrometry (GC-MS) for sterols and terpenoids (mono-, sesqui-, tri-), and high-performance liquid chromatography (HPLC) with UV and mass spectrometric (MS) detection for flavonoids
Embalagem
Outras notas
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Acute 1 - Aquatic Chronic 2 - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
111.2 °F - closed cup
Ponto de fulgor (°C)
44 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
-Cymene; Linalool; Menthol; α-Terpineol; Menthyl acetate
Protocolos
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
-Cymene; (−)-Menthone; α-Terpineol, natural, ≥96%, FCC, FG; Terpinolene; β-Bourbonene; 1-Octen-3-ol; β-Caryophyllene; Linalool; α-Terpinene; (−)-Menthol
Cymene; 4,5,6,7-Tetrahydro-3,6-dimethylbenzofuran; Linalool; Menthol; Menthone; Menthyl acetate; Germacrene D; Bicyclogermacrene; Thymol
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica