64306
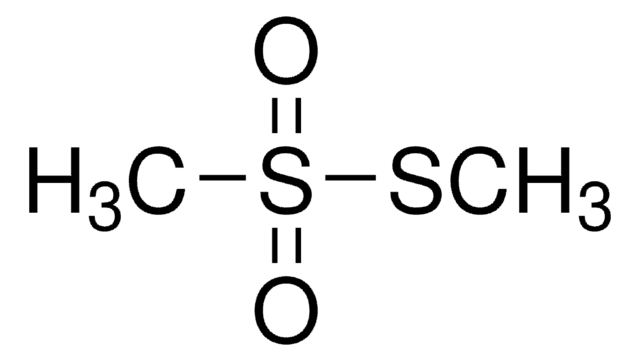
S-Methyl methanethiosulfonate
purum, ≥98.0% (GC)
Sinônimo(s):
S-Methyl thiomethanesulfonate, MMTS
About This Item
Produtos recomendados
grau
purum
Nível de qualidade
Ensaio
≥98.0% (GC)
índice de refração
n20/D 1.513 (lit.)
n20/D 1.513
p.e.
69-71 °C/0.4 mmHg (lit.)
solubilidade
chloroform: 750mg + 5 ml Chloroform mg/mL, colorless to light greenish-yellow
densidade
1.337 g/mL at 20 °C
1.337 g/mL at 25 °C (lit.)
grupo funcional
disulfide
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CSS(C)(=O)=O
InChI
1S/C2H6O2S2/c1-5-6(2,3)4/h1-2H3
chave InChI
XYONNSVDNIRXKZ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Modification of Thiol Enzymes: S-methyl methanethiosulfonate (MMTS) offers a unique method for the modification of thiol enzymes and redox-regulated proteins, providing potential applications in biochemical research focused on enzyme regulation and redox biology (Makarov et al., 2019).
- Sensor Development for Protease Activity: S-methyl methanethiosulfonate is used as a blocking reagent on the structural transitions of papain-like cysteine proteases, which supports its utility in sensor development, allowing for the detection and analysis of protease activity in various biological processes (Markovic et al., 2023).
- Agricultural Pathogen Control: Research evaluating S-methyl methanethiosulfonate as a late blight inhibitor highlights its potential as a broad-range toxin against plant pathogens, suggesting applications in agriculture for the management of crop diseases (Joller et al., 2020).
Atenção
Outras notas
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
188.6 °F - closed cup
Ponto de fulgor (°C)
87 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica