62155
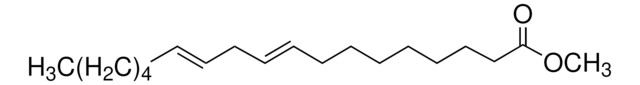
Methyl linolelaidate
analytical standard
Sinônimo(s):
Linolelaidic acid methyl ester, Methyl trans,trans-9,12-octadecadienoate
About This Item
Produtos recomendados
grau
analytical standard
Nível de qualidade
Ensaio
≥99.0% (GC)
prazo de validade
limited shelf life, expiry date on the label
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
formato
neat
grupo funcional
ester
Condições de expedição
ambient
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
CCCCC\C=C\C\C=C\CCCCCCCC(=O)OC
InChI
1S/C19H34O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19(20)21-2/h7-8,10-11H,3-6,9,12-18H2,1-2H3/b8-7+,11-10+
chave InChI
WTTJVINHCBCLGX-ZDVGBALWSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Embalagem
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Protocolos
gc-analysis-of-a-37-component-fame-mix-g004278
GC Analysis of a 37-Component FAME Mix on Omegawax® (15 m x 0.10 mm I.D., 0.10 μm), Fast GC Analysis
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica