17755
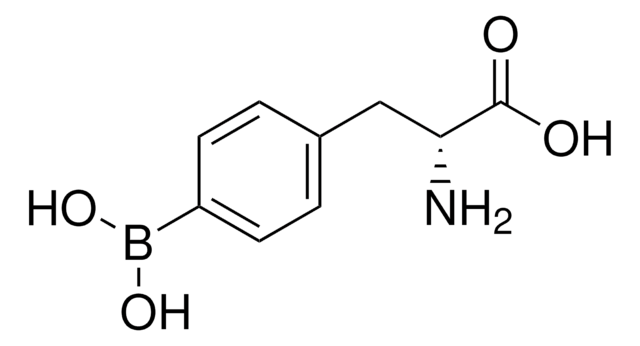
4-Borono-L-phenylalanine
≥95.0% (HPLC)
Sinônimo(s):
4-Dihydroxyboryl-L-phenylalanine, L-BPA
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C9H12BNO4
Número CAS:
Peso molecular:
209.01
Beilstein:
4458616
Número MDL:
Código UNSPSC:
12352209
ID de substância PubChem:
NACRES:
NA.21
Produtos recomendados
Nível de qualidade
Ensaio
≥95.0% (HPLC)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
N[C@@H](Cc1ccc(cc1)B(O)O)C(O)=O
InChI
1S/C9H12BNO4/c11-8(9(12)13)5-6-1-3-7(4-2-6)10(14)15/h1-4,8,14-15H,5,11H2,(H,12,13)/t8-/m0/s1
chave InChI
NFIVJOSXJDORSP-QMMMGPOBSA-N
Aplicação
4-Borono-L-phenylalanine can be used as a building block in solid-phase peptide synthesis. It can also be used to synthesize substituted triazine derivatives as potential tryptophan hydroxylase inhibitors via Suzuki cross-coupling reaction using palladium as a catalyst.
Outras notas
Tyrosine analogue; employed for treatment of melanom cells by boron neutron capture therapy
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Romina F Aromando et al.
Oral oncology, 46(5), 355-359 (2010-03-24)
Mast cell (MC) activation in the hamster cheek pouch cancerization model is associated with the increase in tumor cell proliferation, mediated in turn by tryptase, a protease released from mast cell granules after activation. Tryptase induces tumor cell proliferation through
T Fujimoto et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 69(12), 1713-1716 (2011-03-01)
Clear cell sarcoma (CCS), a rare malignant tumor with a predilection for young adults, is of poor prognosis. Recently however, boron neutron capture therapy (BNCT) with the use of p-borono-L-phenylalanine (BPA) for malignant melanoma has provided good results. CCS also
Ana J Molinari et al.
Radiation research, 177(1), 59-68 (2011-10-11)
We previously demonstrated the efficacy of BNCT mediated by boronophenylalanine (BPA) to treat tumors in a hamster cheek pouch model of oral cancer with no normal tissue radiotoxicity and moderate, albeit reversible, mucositis in precancerous tissue around treated tumors. It
Andrea Wittig et al.
Radiation research, 172(4), 493-499 (2009-09-24)
In boron neutron capture therapy, the absorbed dose from the (10)B(n,alpha)(7)Li reaction depends on the (10)B concentration and (10)B distribution in the irradiated volume. Thus compounds used in BNCT should have tumor-specific uptake and low accumulation in normal tissues. This
Tsubasa Watanabe et al.
BMC cancer, 16(1), 859-859 (2016-11-09)
Boron neutron capture therapy (BNCT) is a cellular-level particle radiation therapy that combines the selective delivery of boron compounds to tumour tissue with neutron irradiation. L-p-Boronophenylalanine (L-BPA) is a boron compound now widely used in clinical situations. Determination of the
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica