429700
Leptin, Human, Recombinant, E. coli
Sinônimo(s):
Leptin, Human, Recombinant, E. coli, rhOB
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Produtos recomendados
esterilidade
non-sterile
Nível de qualidade
Ensaio
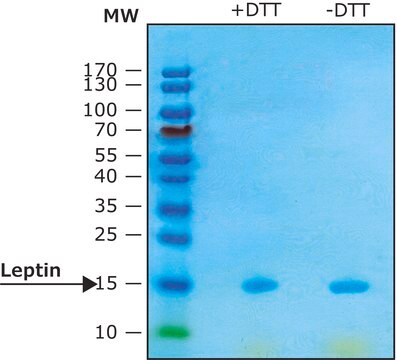
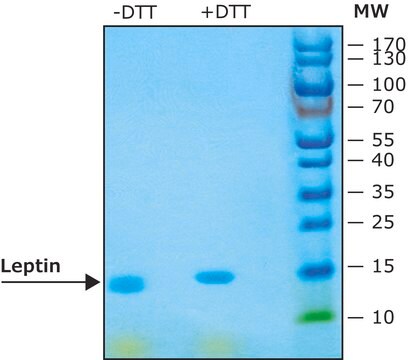
≥97% (SDS-PAGE)
forma
lyophilized
fabricante/nome comercial
Calbiochem®
condição de armazenamento
OK to freeze
Impurezas
≤1.0 EU/μg Endotoxin (EU/μg leptin)
Condições de expedição
ambient
temperatura de armazenamento
−70°C
Descrição geral
Recombinant, human leptin expressed in E. coli. Leptin was originally identified as a protein product of the mouse obese gene. Mice with mutations in the obese gene that block the synthesis of leptin have been found to be obese and diabetic and to have reduced activity, metabolism and body temperature. cDNA clones encoding leptin have been isolated from human, simian, mouse and rat cells. Human leptin shares approximately 84% sequence identity with the mouse protein. Human leptin cDNA encodes a 167 amino acid residue protein with a 21 amino acid signal sequence that is cleaved to yield the 146 amino acid mature protein. The expression of leptin mRNA has been shown to be restricted to adipose tissue.
A high-affinity receptor for leptin (OB-R) with homology to gp130 and the G-CSF receptor was subsequently cloned. The OB-R cytoplasmic domain transduces the leptin signal through the JAK-STAT pathway. Although OB-R mRNA was initially shown to be expressed predominantly in the choroid plexus and in the hypothalamus, more recent data also revealed the expression of this receptor in endothelial cells (Ecs). Furthermore, the angiogenic activity of leptin has been demonstrated both in vitro and in vivo, suggesting a physical mechanism whereby leptin-induced angiogenesis may facilitate increased energy expenditure.
A high-affinity receptor for leptin (OB-R) with homology to gp130 and the G-CSF receptor was subsequently cloned. The OB-R cytoplasmic domain transduces the leptin signal through the JAK-STAT pathway. Although OB-R mRNA was initially shown to be expressed predominantly in the choroid plexus and in the hypothalamus, more recent data also revealed the expression of this receptor in endothelial cells (Ecs). Furthermore, the angiogenic activity of leptin has been demonstrated both in vitro and in vivo, suggesting a physical mechanism whereby leptin-induced angiogenesis may facilitate increased energy expenditure.
Recombinant, human leptin expressed in E. coli. Native leptin is a product of the obese (ob) gene that serves as a ligand for the OB receptor (OB-R). Mice with mutations of the ob gene have been found to be obese and diabetic and to have reduced activity, metabolism, and body temperature. Reported to reduce hepatic glucose production by blocking phosphoenolpyruvate synthesis. Note: Following complete dissolution in 15 mM HCl, add 7.5 mM sterile NaOH and bring the pH to approximately 5.2.
Ações bioquímicas/fisiológicas
ED₅₀ = 0.4-2 ng/ml as measured by its ability to induce proliferation of leptin-dependent rOB-R transfected murine BAF3 cells
Advertência
Toxicity: Standard Handling (A)
forma física
Lyophilized from a sterile filtered solution in PBS.
Reconstituição
To reconstitute lyophilized leptin, add 15 mM sterile HCl (0.5 ml/1 mg vial or 2.5 ml/5 mg vial) to the vial. After the protein is completely dissolved, add 7.5 mM sterile NaOH (0.3 ml/1mg vial or 1.5 ml/5 mg vial) to bring the pH to ~5.2. Lyophilized samples are stable for at least six months at -70°C. Upon reconstitution, this cytokine can be stored under sterile conditions at 4°C for one month or at -70°C for three months without detectable loss of activity. Avoid repeated freeze/thaw cycles of reconstituted solutions.
Outras notas
Anderwald, C., et al. 2002. Mol. Endocrinol.16, 1612.
Ookuma, M., et al. 1998. Diabetes 47, 219.
Campfield, L.A., et al. 1995. Science 269, 546.
Halaas, J.L., et al. 1995. Science 269, 543.
Pelleymounter, M.A., et al. 1995. Science 269, 540.
Zhang, Y., et al. 1994. Nature372, 425.
Ookuma, M., et al. 1998. Diabetes 47, 219.
Campfield, L.A., et al. 1995. Science 269, 546.
Halaas, J.L., et al. 1995. Science 269, 543.
Pelleymounter, M.A., et al. 1995. Science 269, 540.
Zhang, Y., et al. 1994. Nature372, 425.
Informações legais
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica