857324P
Avanti
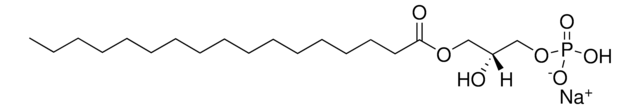
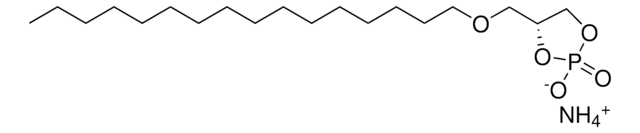
17:0 Cyclic LPA
Avanti Research™ - A Croda Brand 857324P, powder
Sinônimo(s):
1-heptadecanoyl-glycero-2,3-cyclic-phosphate (ammonium salt)
About This Item
Produtos recomendados
Ensaio
>99% (TLC)
Formulário
powder
embalagem
pkg of 1 × 1 mg (857324P-1mg)
fabricante/nome comercial
Avanti Research™ - A Croda Brand 857324P
tipo de lipídio
phospholipids
cardiolipins
Condições de expedição
dry ice
temperatura de armazenamento
−20°C
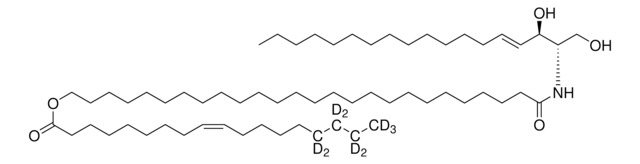
cadeia de caracteres SMILES
O=P1([O-])O[C@H](COC(CCCCCCCCCCCCCCCC)=O)CO1.[NH4+]
InChI
1S/C20H39O6P.H3N/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-20(21)24-17-19-18-25-27(22,23)26-19;/h19H,2-18H2,1H3,(H,22,23);1H3/t19-;/m1./s1
Descrição geral
Aplicação
Ações bioquímicas/fisiológicas
Interestingly, many of these cellular responses caused by cPA oppose those of LPA despite the activation of apparently overlapping receptor populations.
Embalagem
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Ponto de fulgor (°F)
No data available
Ponto de fulgor (°C)
No data available
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lamentamos, não temos COA para este produto disponíveis online no momento.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica