W237809
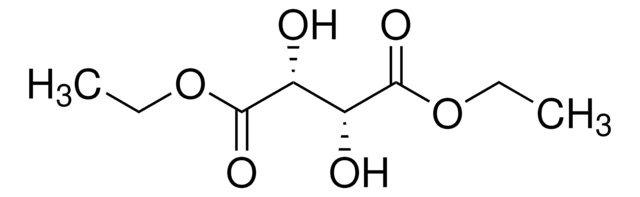
Diethyl L-tartrate
≥99%, FG
Sinônimo(s):
(+)-Diethyl L-tartrate, L-(+)-Tartaric acid diethyl ester
About This Item
Fragrance grade
Kosher
meets purity specifications of JECFA
Produtos recomendados
fonte biológica
synthetic
Nível de qualidade
grau
FG
Fragrance grade
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
conformidade reg.
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
Ensaio
≥99%
atividade óptica
[α]20/D +8.5°, neat
índice de refração
n20/D 1.446 (lit.)
p.e.
280 °C (lit.)
densidade
1.204 g/mL at 25 °C (lit.)
aplicação(ões)
flavors and fragrances
Documentação
see Safety & Documentation for available documents
alérgeno alimentar
no known allergens
alérgeno de fragrância
no known allergens
Organoléptico
fruity; wine-like
cadeia de caracteres SMILES
CCOC(=O)[C@H](O)[C@@H](O)C(=O)OCC
InChI
1S/C8H14O6/c1-3-13-7(11)5(9)6(10)8(12)14-4-2/h5-6,9-10H,3-4H2,1-2H3/t5-,6-/m1/s1
chave InChI
YSAVZVORKRDODB-PHDIDXHHSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Synthesis of l-threitol-based crown ethers and their application as enantioselective phase transfer catalyst in Michael additions.: This study synthesizes l-threitol-based crown ethers using diethyl ʟ-tartrate and explores their efficacy as enantioselective phase transfer catalysts in Michael additions, highlighting their potential in asymmetric synthesis (Rapi et al., 2017).
- A facile approach for the synthesis of C13-C24 fragments of maltepolides A, C and D.: This research demonstrates a novel synthesis method for C13-C24 fragments of maltepolides A, C, and D using diethyl ʟ-tartrate, facilitating the study and development of these bioactive compounds (Rao & Srihari, 2016).
- Development of diacyltetrol lipids as activators for the C1 domain of protein kinase C.: This research introduces diacyltetrol lipids synthesized from diethyl ʟ-tartrate, which act as activators for the C1 domain of protein kinase C, offering insights into signal transduction and therapeutic applications (Mamidi et al., 2012).
- Total synthesis of broussonetine F: the orthoamide Overman rearrangement of an allylic diol.: The paper presents the total synthesis of broussonetine F, utilizing diethyl ʟ-tartrate in an orthoamide Overman rearrangement, showcasing a novel synthetic route for complex natural products (Hama et al., 2011).
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
199.4 °F - closed cup
Ponto de fulgor (°C)
93 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica