P39605
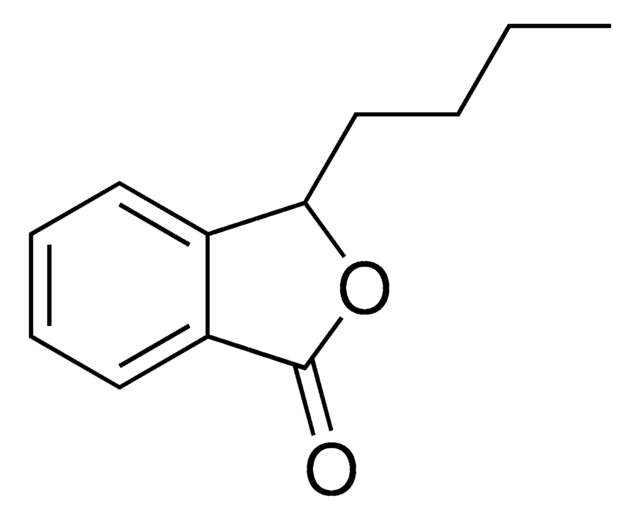
Phthalide
98%
Sinônimo(s):
1-Isobenzofuranone
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C8H6O2
Número CAS:
Peso molecular:
134.13
Beilstein:
114632
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
Formulário
powder
p.e.
290 °C (lit.)
pf
71-74 °C (lit.)
cadeia de caracteres SMILES
O=C1OCc2ccccc12
InChI
1S/C8H6O2/c9-8-7-4-2-1-3-6(7)5-10-8/h1-4H,5H2
chave InChI
WNZQDUSMALZDQF-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
305.6 °F - closed cup
Ponto de fulgor (°C)
152 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Fangrui Zhong et al.
Journal of the American Chemical Society, 134(24), 10222-10227 (2012-05-25)
Phthalides were used for the first time in the allylic alkylation reactions with MBH carbonates for the creation of chiral 3,3-disubstituted phthalides. Highly enantioselective regiodivergent synthesis of γ-selective or β-selective allylic alkylation products was achieved by employing bifunctional chiral phosphines
Yun Wei et al.
Journal of chromatography. A, 1284, 53-58 (2013-03-15)
The phthalide compounds of Chuanxiong rhizoma including senkyunolide A, levistolide A, Z-ligustilide and 3-butylidenephthalide, have been reported as the biologically active compounds because of their therapeutic effects. In this work, online high-speed counter-current chromatography coupled with semi-preparative liquid chromatography instrument
Bin Xiao et al.
Bioorganic & medicinal chemistry, 20(16), 4954-4961 (2012-07-24)
On the basis of a marine fungal phthalide (paecilocin A) skeleton, we synthesized 20 analogs and evaluated them for peroxisome proliferator-activated receptor gamma (PPAR-γ) binding and activation. Among these analogs, 6 and 7 had significant PPAR-γ binding activity, and 7
Chang-Yin Li et al.
Journal of pharmaceutical and biomedical analysis, 55(1), 146-160 (2011-02-01)
In this work, the metabolite profiles of Danggui Buxue Tang (DBT) in rat bile and plasma were qualitatively described, and the possible metabolic pathways of DBT were subsequently proposed. Emphasis was put on correlative analysis of metabolite profiling in different
Song-Hwa Chae et al.
Journal of agricultural and food chemistry, 59(15), 8193-8198 (2011-07-07)
The residual contact toxicity of three benzofuranoids (Z)-butylidenephthalide (1), (3S)-butylphthalide (2), and (Z)-ligustilide (3) identified in the rhizome of Cnidium officinale (Apiaceae) to B- and Q-biotype females of Bemisia tabaci was evaluated using a leaf-dip bioassay. Results were compared with
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica