About This Item
Fórmula linear:
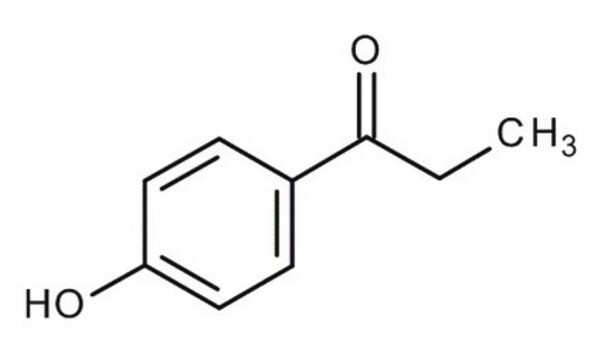
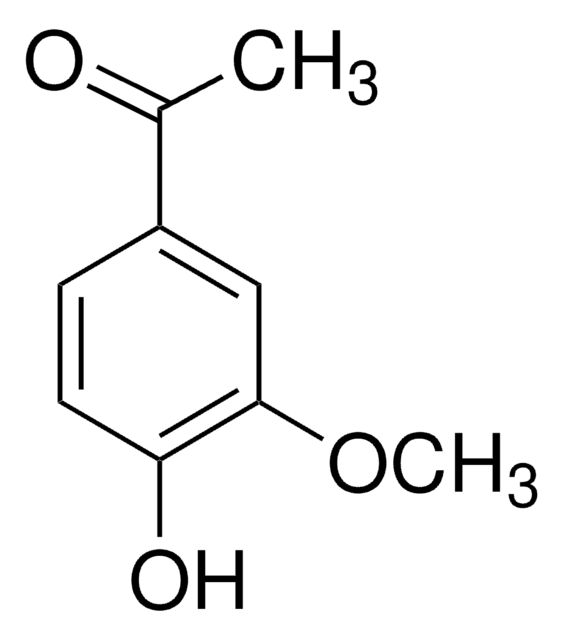
HOC6H4COC2H5
Número CAS:
Peso molecular:
150.17
Beilstein:
907511
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
pf
147.5-148.5 °C (lit.)
cadeia de caracteres SMILES
CCC(=O)c1ccc(O)cc1
InChI
1S/C9H10O2/c1-2-9(11)7-3-5-8(10)6-4-7/h3-6,10H,2H2,1H3
chave InChI
RARSHUDCJQSEFJ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
p-Hydroxypropiophenone effects on azo dye-induced alterations in mouse hepatic cells: light and electron microscopic study.
N J Unakar
Journal of the National Cancer Institute, 44(4), 873-891 (1970-04-01)
A Tanner et al.
Journal of bacteriology, 182(23), 6565-6569 (2000-11-14)
An arylketone monooxygenase was purified from Pseudomonas putida JD1 by ion exchange and affinity chromatography. It had the characteristics of a Baeyer-Villiger-type monooxygenase and converted its substrate, 4-hydroxyacetophenone, into 4-hydroxyphenyl acetate with the consumption of one molecule of oxygen and
Zack E Bryant et al.
Bioorganic & medicinal chemistry letters, 21(3), 912-915 (2011-01-14)
A series of ethacrynic acid analogues, lacking the α,β-unsaturated carbonyl unit, was synthesized and subsequently evaluated for their ability to inhibit the migration of human breast cancer cells, Hs578Ts(i)8 as well as of human prostate cancer cells, C4-2B. These cell
R Cizmáriková et al.
Ceskoslovenska farmacie, 42(2), 82-85 (1993-04-01)
Within the relationship of the structure and effect of new beta-adrenolytic agents derivatived from p-hydroxyacetophenone and p-hydroxypropiophenone with a propoxymethyl group in the lipophilic part of the molecule and with a propanamine, a butanamine and a pyrrolidine in the side-chain
R Cizmáriková et al.
Ceska a Slovenska farmacie : casopis Ceske farmaceuticke spolecnosti a Slovenske farmaceuticke spolecnosti, 43(5), 226-228 (1994-10-01)
The present paper carries out the pharmacological evaluation of 4-(2-hydroxy-3-isopropylaminopropoxy)-3-(alkoxymethyl) propiophenones with an ethoxy, propoxy and butoxy-group, whose structures are typical of the blockers of beta-adrenergic receptors. In the above-mentioned compounds the anticalcium effect on the frequency and the amplitude
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica