H17082
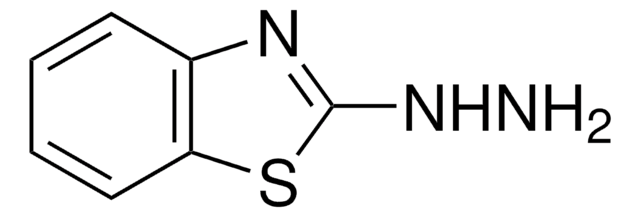
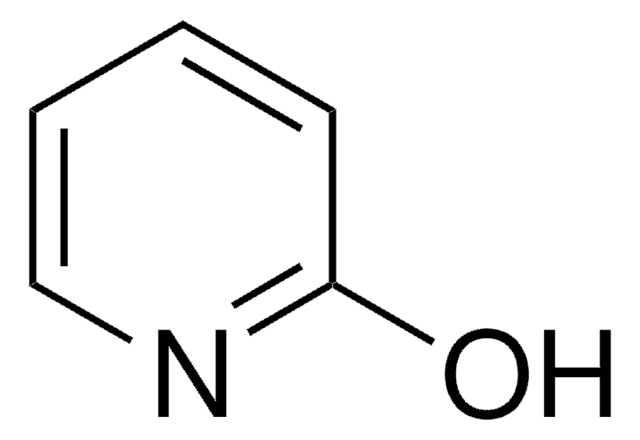
2-Hydrazinopyridine
97%
Sinônimo(s):
2-Pyridylhydrazine
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C5H7N3
Número CAS:
Peso molecular:
109.13
Beilstein:
109984
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
p.e.
90-92 °C/1 mmHg (lit.)
pf
41-44 °C (lit.)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
NNc1ccccn1
InChI
1S/C5H7N3/c6-8-5-3-1-2-4-7-5/h1-4H,6H2,(H,7,8)
chave InChI
NWELCUKYUCBVKK-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
- Enhanced Metal Removal: Utilizing Schiff base functionalized dialdehyde starch, derived from 2-hydrazinopyridine, demonstrated significant potential in enhancing the removal of Cu(II) from solutions. The study included preparation methodologies, performance evaluations, and DFT calculations, showcasing its efficacy in water treatment technologies (Liang et al., 2024).
- Dual Sensing Probe Development: A novel dicyanisophorone-based probe, incorporating 2-hydrazinopyridine, was developed for the dual sensing of Zn(2+) and Cd(2+) via near-infrared fluorescence. This advancement aids in the detection and analysis of heavy metals in various environmental and biological samples (Yan et al., 2023).
- Active Site Analysis in Lysyl Oxidase: The study provided insights into the spatial arrangement of active site components in Lysyl Oxidase-like 2, including the role of 2-hydrazinopyridine, which is critical for understanding the enzyme′s mechanism and potential therapeutic applications (Meier et al., 2022).
- Structural Analysis of Lysyl Oxidase: Research focused on the predicted 3D structure of the amine oxidase domain of Lysyl Oxidase-Like 2, exploring the interaction dynamics facilitated by 2-hydrazinopyridine. This contributes significantly to the field of molecular biology and enzyme function analysis (Meier et al., 2022).
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
230.0 °F - closed cup
Ponto de fulgor (°C)
110 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
E S H El Ashry et al.
Nucleosides, nucleotides & nucleic acids, 23(3), 567-580 (2004-04-29)
Reaction of 2-hydrazinopyridine (1) with D-xylose, D-galactose, D-glucose and D-fructose afforded the corresponding hydrazones mainly in the acyclic forms 2, 3, 6 and 11 with minor amounts of the cyclic structures. Oxidative cyclization of the hydrazones with bromine in methanol
C M Wilmot et al.
Biochemistry, 36(7), 1608-1620 (1997-02-18)
The crystal structure of the complex between the copper amine oxidase from Escherichia coli (ECAO) and a covalently bound inhibitor, 2-hydrazinopyridine, has been determined to a resolution of 2.0 A. The inhibitor covalently binds at the 5 position of the
G De Matteis et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 4(3), 348-353 (1999-08-10)
Bovine serum amine oxidase (BSAO) reacts with 2-hydrazinopyridine, which binds the organic co-factor 2,4,5-trihydroxyphenylalanine quinone, forming a band at 435 nm. The band shifts to 526 nm around 60 degrees C, to 415 nm upon denaturation, but only shifts to
Conserved tyrosine-369 in the active site of Escherichia coli copper amine oxidase is not essential.
J M Murray et al.
Biochemistry, 40(43), 12808-12818 (2001-10-24)
Copper amine oxidases are homodimeric enzymes that catalyze two reactions: first, a self-processing reaction to generate the 2,4,5-trihydroxyphenylalanine (TPQ) cofactor from an active site tyrosine by a single turnover mechanism; second, the oxidative deamination of primary amine substrates with the
Minae Mure et al.
Biochemistry, 44(5), 1583-1594 (2005-02-03)
Adduct I (lambda(max) at approximately 430 nm) formed in the reaction of 2-hydrazinopyridine (2HP) and the TPQ cofactor of wild-type Escherichia coli copper amine oxidase (WT-ECAO) is stable at neutral pH, 25 degrees C, but slowly converts to another spectroscopically
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica