D108405
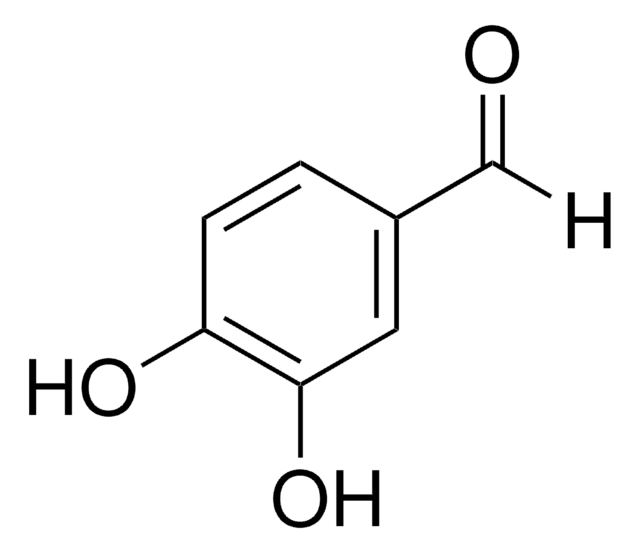
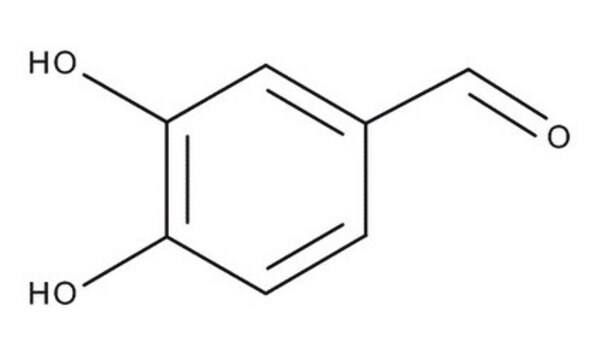
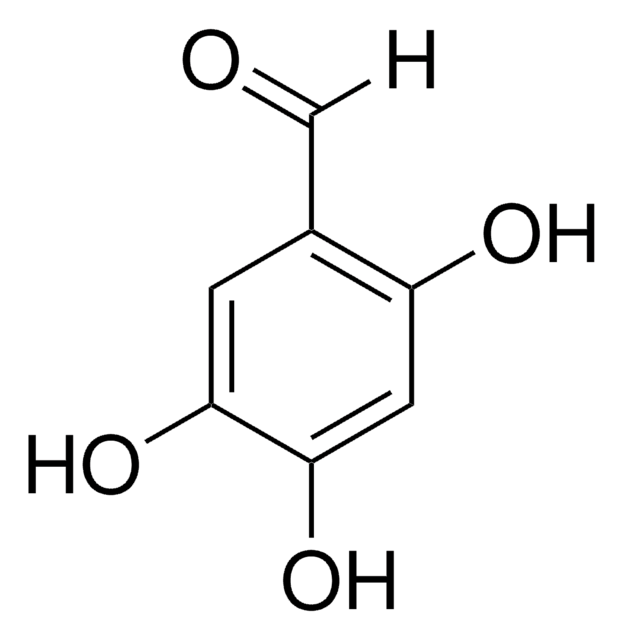
3,4-Dihydroxybenzaldehyde
97%
Sinônimo(s):
Protocatechualdehyde
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
(HO)2C6H3CHO
Número CAS:
Peso molecular:
138.12
Beilstein:
774381
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
forma
powder
pf
150-157 °C (lit.)
cadeia de caracteres SMILES
Oc1ccc(C=O)cc1O
InChI
1S/C7H6O3/c8-4-5-1-2-6(9)7(10)3-5/h1-4,9-10H
chave InChI
IBGBGRVKPALMCQ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
3,4-Dihydroxybenzaldehyde can be used as a reactant for the synthesis of:
- Copolymers containing poly(p-phenylenevinylene) chromophore to be used in light-emitting electrochemical cell.
- 2-Arylbenzothiazoles with potential application as anti-cancer agents against human colon cancer cells.
- Variety of thiazolidin-4-one ring systems having antimicrobial activity.
- Bis-Schiff bases of isatins which can be used as antiglycating agents.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Synthesis of bis-Schiff bases of isatins and their antiglycation activity.
Khan KM, et al.
Bioorganic & Medicinal Chemistry, 17(22), 7795-7801 (2009)
Synthesis of new bioactive venlafaxine analogs: Novel thiazolidin-4-ones as antimicrobials.
Kavitha CV, et al.
Bioorganic & Medicinal Chemistry, 14(7), 2290-2299 (2006)
Synthesis and electroluminescence of novel copolymers containing crown ether spacers.
Sun Q, et al.
Journal of Materials Chemistry, 13(4), 800-806 (2003)
Catriona G Mortimer et al.
Journal of medicinal chemistry, 49(1), 179-185 (2006-01-06)
A series of new 2-phenylbenzothiazoles has been synthesized on the basis of the discovery of the potent and selective in vitro antitumor properties of 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (8n; GW 610, NSC 721648). Synthesis of analogues substituted in the benzothiazole ring was achieved
Narsimha Mamidi et al.
The journal of physical chemistry. B, 116(35), 10684-10692 (2012-08-07)
Diacylglycerol (DAG) regulates a broad range of cellular functions including tumor promotion, apoptosis, differentiation, and growth. Thus, the DAG-responsive C1 domain of protein kinase C (PKC) isoenzymes is considered to be an attractive drug target for the treatment of cancer
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica