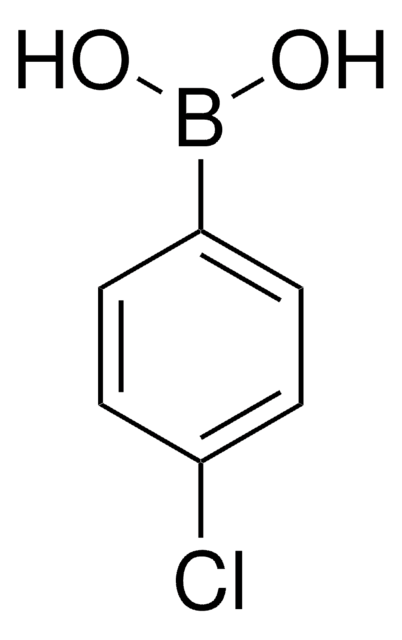
B75956
4-Bromophenylboronic acid
≥95.0%
Sinônimo(s):
(p-Bromophenyl)boronic acid, 4-Bromobenzeneboronic acid, 4-Bromophenylboric acid, p-Bromobenzeneboronic acid, p-Bromophenylboric acid, NSC 25407
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥95.0%
95%
forma
crystals
pf
284-288 °C (lit.)
cadeia de caracteres SMILES
OB(O)c1ccc(Br)cc1
InChI
1S/C6H6BBrO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
chave InChI
QBLFZIBJXUQVRF-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Palladium catalyzed Suzuki-Miyaura cross-couplings
- Pd(II)-catalyzed diastereoselective conjugate additions
- Palladium-catalyzed stereoselective Heck-type reaction of allylic esters with arylboronic acids
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides
- Pd-catalyzed arylative cyclization of alkyne-tethered enals or enones via carbopalladation of alkynes
- Copper-catalyzed cross-couplings
Reagent used in Preparation of
- Gallate-based obovatol analogs with potential anti-tumor activity
- Protein modulators and enzymatic and kinase inhibitors
Outras notas
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)