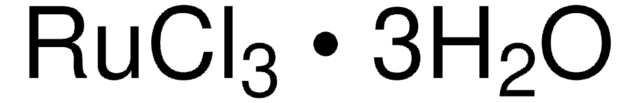931578
Cloreto de rutênio (III)
≥99.9% trace metals basis
Sinônimo(s):
Ruthenium trichloride, Trichlororuthenium hydrate
About This Item
Produtos recomendados
grau
for analytical purposes
Nível de qualidade
Ensaio
≥99.9% trace metals basis
Formulário
powder
Impurezas
≤1000.0 ppm (trace metals analysis)
solubilidade
acetone: soluble ((lit.))
ethanol: soluble
water: soluble
densidade
3.11 g/cm3
cadeia de caracteres SMILES
O.Cl[Ru](Cl)Cl
InChI
1S/3ClH.H2O.Ru/h3*1H;1H2;/q;;;;+3/p-3
chave InChI
BIXNGBXQRRXPLM-UHFFFAOYSA-K
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Industrially, ruthenium trichloride hydrate is produced by dissolving ruthenium oxides in hydrochloric acid. The hydrated salt is obtained by recrystallization.
Aplicação
Another common application of ruthenium chloride hydrate is as a precursor for single-atom catalysts. For example, scientists have used ruthenium chloride hydrate for the synthesis of ruthenium single-atom-doped ZrO2 particles to catalyze nitrogen fixation and for the synthesis of ruthenium single-atom-doped MXenes to catalyze hydrogen evolution. A third common application of ruthenium chloride hydrate is in the synthesis of metal alloys, like PtRuIr, or PtRuFe, which are investigated for electrocatalysis, usually the oxidation of simple organics like methanol or formic acid.
For use in all these applications, also consider our higher-purity ruthenium chloride hydrate, 463779, with trace metals purity greater than 99.98%, which offers the best reproducibility and purity.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Código de classe de armazenamento
8A - Combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica