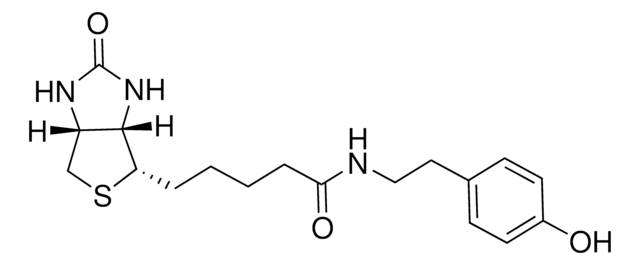
914924
3-[2-N-(Biotinyl)aminoethyldithio]propanoic acid
≥95%
Sinônimo(s):
3-((2-(5-((3aS,4S,6aR)-2-Oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamido)ethyl)disulfaneyl)propanoic acid, Biotin-SS-COOH, Cleavable biotin linker
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥95%
forma
powder
pf
172-175 °C
temperatura de armazenamento
−20°C
InChI
1S/C15H25N3O4S3/c19-12(16-6-8-25-24-7-5-13(20)21)4-2-1-3-11-14-10(9-23-11)17-15(22)18-14/h10-11,14H,1-9H2,(H,16,19)(H,20,21)(H2,17,18,22)/t10-,11-,14-/m0/s1
chave InChI
LUKYYZVIDAWYMZ-MJVIPROJSA-N
Aplicação
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Outras notas
A Mechanism-Based ICAT Strategy for Comparing Relative Expression and Activity Levels of Glycosidases in Biological Systems
Dissociation-independent selection of high-affinity anti-hapten phage antibodies using cleavable biotin-conjugated haptens
produto relacionado
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lamentamos, não temos COA para este produto disponíveis online no momento.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica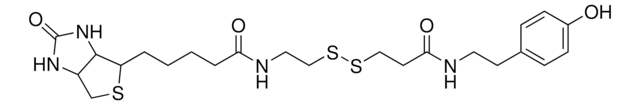

![Spiro[9H-fluorene-9,9′-[9H]xanthene]-2,7-diamine](/deepweb/assets/sigmaaldrich/product/structures/307/234/46c07f0c-9242-4c0b-8994-8121690da3c9/640/46c07f0c-9242-4c0b-8994-8121690da3c9.png)