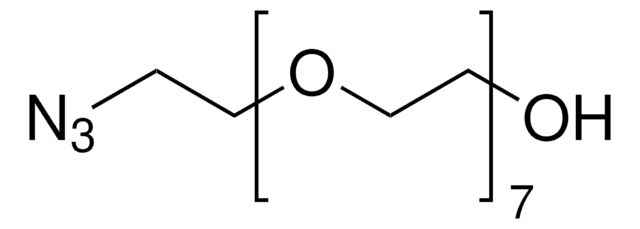
900935
Poly(ethylene glycol) α-hydroxy-ω-azido terminated
average Mn 5,000
Sinônimo(s):
α-Hydroxy-ω-azido-PEG, PEG-Azide, Polyethylene glycol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
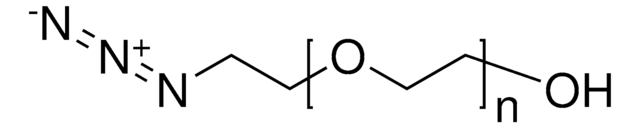
N3CH2CH2(OCH2CH2)nOH
Código UNSPSC:
51171641
NACRES:
NA.23
Produtos recomendados
Formulário
powder or chunks
Nível de qualidade
peso molecular
Mn 4000-6000 (by NMR)
average Mn 5,000
cor
white to off-white
temperatura de armazenamento
−20°C
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
α-Hydroxy-ω-azido terminated-poly(ethylene glycol) is a heterobifunctional PEG derivative that can be used to modify peptides, proteins, or other bioconjugation chemistry applications. PEGylated materials have found broad use in drug delivery systems, virology, and immunology, as the incorporation of PEG improves pharmacological properties such as increased water solubility, enhanced resistance to degradation (protein hydrolysis), increased circulation half-life, and reduced antigenicity. In addition to PEGylation, this heterobifunctional PEG can also be used to form networks for tissue engineering or drug delivery applications due to its dual reactivity.
Aplicação
α-Hydroxy-ω-azido terminated-poly(ethylene glycol) features two distinct, terminal functional groups: an azide and a hydroxyl group. The terminal azide can undergo copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) or strain promoted azide-alkyne cycloaddition (spAAC), depending on reaction conditions and the identity of the alkyne. In addition, the terminal azide can be reduced to an amine in mild conditions for use in other coupling reactions. The free hydroxyl allows for additional functionalization or a secondary coupling reaction.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lot/Batch Number
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Joseph G Plaks et al.
Bioconjugate chemistry, 26(6), 1104-1112 (2015-05-20)
Approaches that allow bioorthogonal and, in turn, site-specific chemical modification of proteins present considerable opportunities for modulating protein activity and stability. However, the development of such approaches that enable site-selective modification of proteins at multiple positions, including internal sites within
Sabrina M Hodgson et al.
Biomacromolecules, 17(3), 1093-1100 (2016-02-05)
A series of poly(ethylene glycol) (PEG) hydrogels was synthesized using strain-promoted alkyne-azide cycloaddition (SPAAC) between PEG chains terminated with either aza-dibenzocyclooctynes or azide functionalities. The gelation process was found to occur rapidly upon mixing the two components in aqueous solution
Kevin N Sill et al.
Biomacromolecules, 18(6), 1874-1884 (2017-05-06)
Described is the development of a polymeric micelle drug delivery platform that addresses the physical property limitations of many nanovectors. The system employs triblock copolymers comprised of a hydrophilic poly(ethylene glycol) (PEG) block, and two poly(amino acid) (PAA) blocks: a
Ian W Hamley
Biomacromolecules, 15(5), 1543-1559 (2014-04-12)
The remarkable diversity of the self-assembly behavior of PEG-peptides is reviewed, including self-assemblies formed by PEG-peptides with β-sheet and α-helical (coiled-coil) peptide sequences. The modes of self-assembly in solution and in the solid state are discussed. Additionally, applications in bionanotechnology
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)