857661
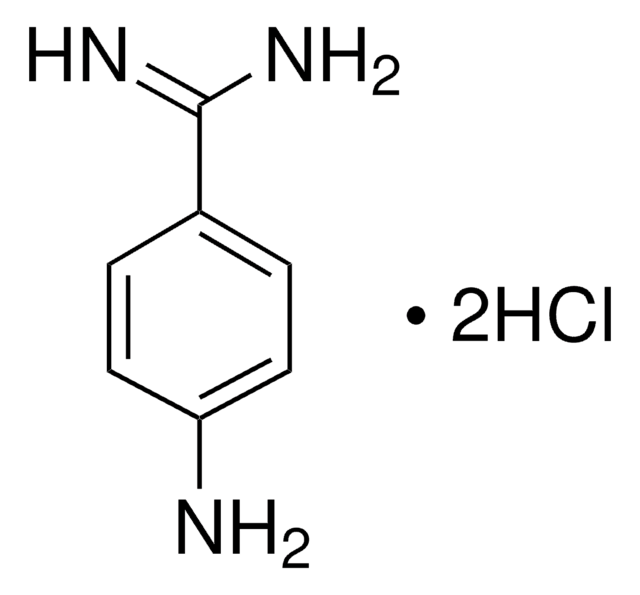
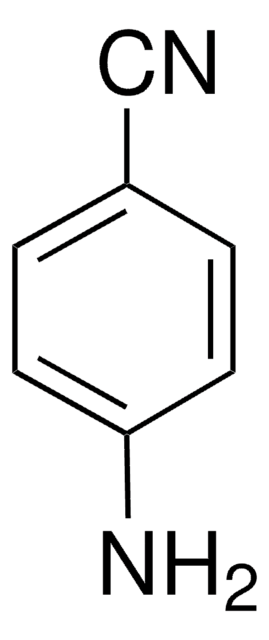
4-Aminobenzamidine dihydrochloride
98%
Sinônimo(s):
p-Aminobenzimidamide dihydrochloride
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
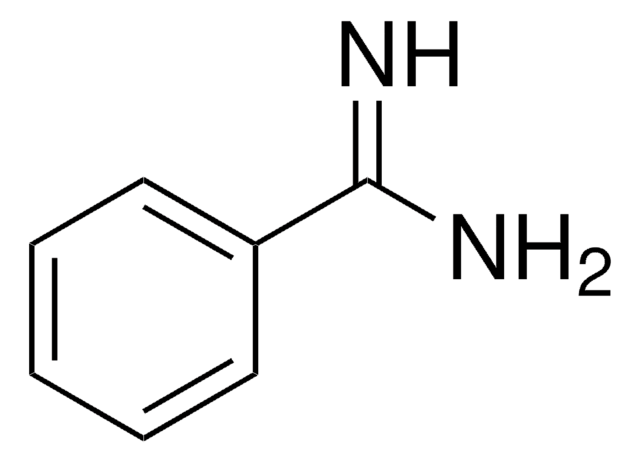
H2NC6H4C(=NH)NH2·2HCl
Número CAS:
Peso molecular:
208.09
Beilstein:
3692927
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
crystals
pf
>300 °C (lit.)
grupo funcional
amine
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
Cl[H].Cl[H].NC(=N)c1ccc(N)cc1
InChI
1S/C7H9N3.2ClH/c8-6-3-1-5(2-4-6)7(9)10;;/h1-4H,8H2,(H3,9,10);2*1H
chave InChI
GHEHNICLPWTXJC-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
4-Aminobenzamidine dihydrochloride can be used to synthesize:
- Orally active fibrinogen receptor antagonists based on benzamidines.
- Benzamidine derivatives that are selective and potent serine protease inhibitors.
- Novel pyrrolo [3,2-c] quinolines that are structural analogs of topoisomerase inhibitors such as coralyne and fagaronine.
4-Aminobenzamidine dihydrochloride is used as a ligand in affinity chromatography for purification and immobilization of enzymes.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Biochemical and molecular modeling analysis of the ability of two p-aminobenzamidine-based sorbents to selectively purify serine proteases (fibrinogenases) from snake venoms.
De-Simone S G, et al.
Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 822(1-2), 1-9 (2005)
In vitro blood compatibility of polymeric biomaterials through covalent immobilization of an amidine derivative.
Gouzy M F, et al.
Biomaterials, 25(17), 3493-3501 (2004)
A L Nguyen et al.
Biotechnology and bioengineering, 34(9), 1186-1190 (1989-11-01)
Reactive polymers have been prepared by copolymeriz-ing N-isopropyl acrylamide (NIPAM) with N-acryloxy-succinimide (NASI) or glycidyl methacrylate (GMA). The amino groups of ligands could react with the residues of NASI or GMA and the polymers could be precipitated by temperature and/or
A L Nguyen et al.
Enzyme and microbial technology, 12(9), 663-668 (1990-09-01)
A reactive water-soluble polymer was synthesized by copolymerizing N-isopropylacrylamide and glycidyl acrylate. The reactive polymer could react with the amino groups of enzymes/proteins or other ligands to form an affinity polymer. As a model, the reactive polymer was allowed to
Specific adsorption of serine proteases on coated silica beads substituted with amidine derivatives.
S Khamlichi et al.
Journal of chromatography, 510, 123-132 (1990-06-27)
Amidine derivatives interact with serine proteases, the inhibition being due to interactions between amidine functions and the active sites of the enzymes. Five different types of amidine (substituted or unsubstituted) were coupled to coated silica beads, which had previously been
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica