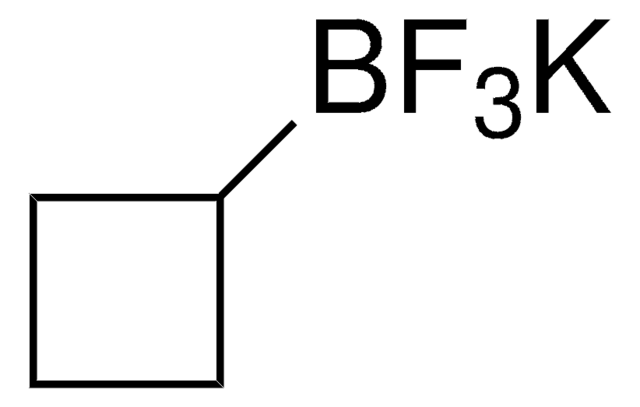
747157
Potassium pentafluroroethyltrifluoroborate
95%
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C2BF8K
Número CAS:
Peso molecular:
225.92
Número MDL:
Código UNSPSC:
12352103
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
95%
Formulário
solid
pf
252-257 °C
grupo funcional
fluoro
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
F[B-](F)(F)C(F)(F)C(F)(F)F.[K+]
InChI
1S/C2BF8.K/c4-1(5,2(6,7)8)3(9,10)11;/q-1;+1
chave InChI
PSJPJAFBTMLFFX-UHFFFAOYSA-N
Categorias relacionadas
Aplicação
Organotrifluoroborate involved in:
Organotrifluoroborates as versatile and stable boronic acid surrogates
- Suzuki Miyaura cross-coupling reactions, and polymerization reactions
- Synthesis of photonic crystals
- Synthesis of sensitizers for dye-sensitized solar cells
- Mannich / diastereoselective hydroamination reaction sequence
Organotrifluoroborates as versatile and stable boronic acid surrogates
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lot/Batch Number
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Gary A Molander et al.
Journal of the American Chemical Society, 130(47), 15792-15793 (2008-11-05)
A method was developed for the hydroboration of alkenyl-containing organotrifluoroborates to generate dibora intermediates. The reactivity differences between organotrifluoroborates and trialkylboranes facilitated the cross-coupling of the borane moiety of these intermediates in a highly chemoselective fashion with aryl halides, leaving
Roberto Grisorio et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(27), 8054-8061 (2010-06-04)
The mechanism of the Suzuki-Heck (SuHe) polymerisation of 2,7-dibromo-9,9-di(n-dodecyl)fluorene (1) with potassium vinyl trifluoroborate (PVTB) for the synthesis of poly(fluorenylene vinylene)s (PFVs) has been investigated. In the first stage, a palladium-catalysed chain-growth AA/B(C)-type polycondensation occurs, as evidenced by the linear
Emilio Alacid et al.
The Journal of organic chemistry, 74(6), 2321-2327 (2009-02-17)
Potassium vinyl and alkenyltrifluoroborates are cross-coupled with aryl and heteroaryl bromides using 1 mol % Pd loading of 4-hydroxyacetophenone oxime derived palladacycle or Pd(OAc)2 as precatalysts, K2CO3 as base, and TBAB as additive and water reflux under conventional or microwave
S. Achelle;
Journal of Polymer Science: Part A, General Papers, 48, 2659-2665 (2010)
Jenny M Baxter Vu et al.
Organic letters, 13(15), 4056-4059 (2011-07-14)
A new two-step synthesis of highly substituted pyrrolidines has been developed. Chiral silane Lewis acid promoted enantioselective Mannich reactions of silyl ketene imines with acylhydrazones may be used to access bishomoallylic benzoic hydrazides that in turn may be cyclized to
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
![Phenyl[3-(trifluoromethyl)phenyl]iodonium triflate ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/424/062/057593f4-e032-4d3e-bed6-6be37c1ae76d/640/057593f4-e032-4d3e-bed6-6be37c1ae76d.png)