742945
TurboBeads™ TEMPO
≥99%
Sinônimo(s):
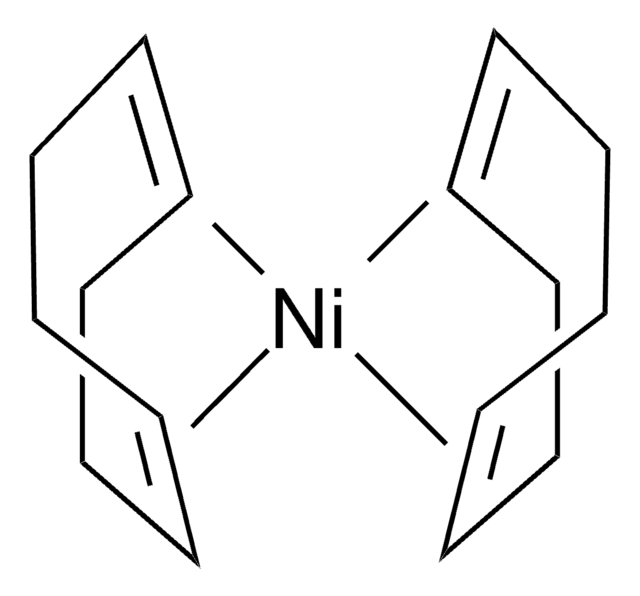
Nano particles, magnetic, TEMPO functionalized
About This Item
Produtos recomendados
linha de produto
TurboBeads™
Ensaio
≥99%
Formulário
powder
composição
carbon content, ≤14 wt. %
adequação da reação
reaction type: solution phase peptide synthesis
reactivity: alcohol reactive
Extensão da rotulagem
≥0.1 mmol/g loading (TEMPO)
magnetização
≥120 emu/g, mass saturation
área da superfície
≥15 m2/g
diâmetro médio
≤50 nm
adequação
conforms to structure for Infrared spectrum
Aplicação
Embalagem
Nota de análise
air-stability:
weight gain in air at 400°C >20 wt.%
weight gain in air at 100°C <3 wt.%
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica