About This Item
Fórmula linear:
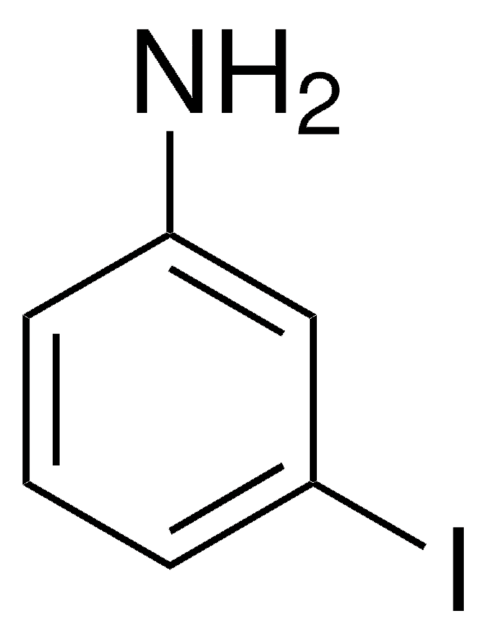
BrC6H3(I)NH2
Número CAS:
Peso molecular:
297.92
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
solid
pf
69-72 °C (lit.)
grupo funcional
bromo
iodo
cadeia de caracteres SMILES
Nc1ccc(Br)cc1I
InChI
1S/C6H5BrIN/c7-4-1-2-6(9)5(8)3-4/h1-3H,9H2
chave InChI
HHTYEQWCHQEJNV-UHFFFAOYSA-N
Descrição geral
4-Bromo-2-iodoaniline is a 2-iodoaniline derivative. It can be prepared by reacting 4-bromoaniline with iodine.
Aplicação
4-Bromo-2-iodoaniline may be used in the following:
- Preparation of quinolone derivatives.
- Synthesis of a resin-bound sulfonamide, which was used as a starting material for the preparation of 2,3,5-trisubstituted indoles.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
T Y Wu et al.
Organic letters, 3(24), 3827-3830 (2001-11-27)
2,3,5-Trisubstituted indoles are synthesized in three steps starting from resin-bound aniline 2. R1 is introduced by a palladium-mediated coupling of the aryl iodide with terminal alkynes followed by intramolecular cyclization to form the indole core. Acylation at C-3 with an
ON SOME HALOGEN DERIVATIVES OF AROMATIC AMINES AND THEIR ANALYSIS. I. 1.
Dains FB, et al.
Journal of the American Chemical Society, 40(6), 930-936 (1918)
Palladium-catalyzed synthesis of 2-quinolone derivatives from 2-iodoanilines.
Cortese NA, et al.
The Journal of Organic Chemistry, 43(15), 2952-2958 (1978)
Shaei Huang et al.
Bioorganic & medicinal chemistry letters, 16(22), 5907-5912 (2006-09-23)
Through a comparison of X-ray co-crystallographic data for 1 and 2 in the Chek1 active site, it was hypothesized that the affinity of the indolylquinolinone series (2) for Chek1 kinase would be improved via C6 substitution into the hydrophobic region
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica