49800
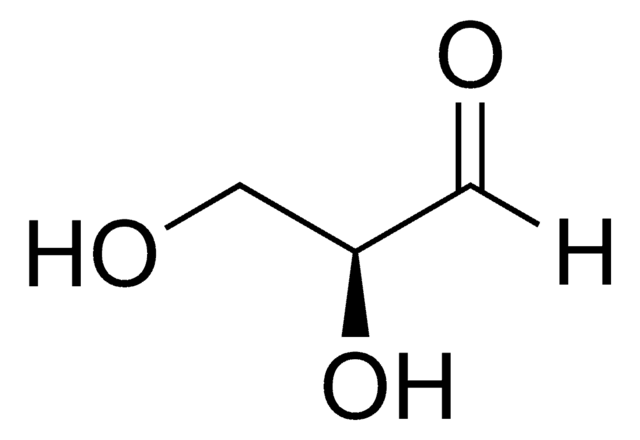
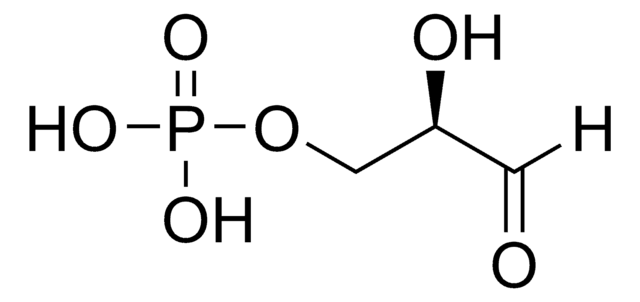
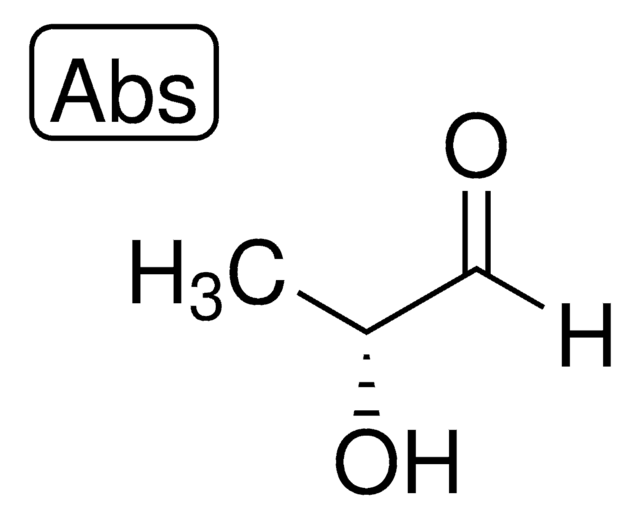
D-(+)-Glyceraldehyde
≥98.0% (HPLC)
Sinônimo(s):
(2R)-2,3-Dihydroxypropanal, Triose
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C3H6O3
Número CAS:
Peso molecular:
90.08
Beilstein:
1720474
Número CE:
Número MDL:
Código UNSPSC:
12352200
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
≥98.0% (HPLC)
Impurezas
≤10% water
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
OC[C@@H](O)C=O
InChI
1S/C3H6O3/c4-1-3(6)2-5/h1,3,5-6H,2H2/t3-/m0/s1
chave InChI
MNQZXJOMYWMBOU-VKHMYHEASA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
D-(+)-Glyceraldehyde can be utilized as a reactant in the synthesis of:
- (S)-homophenylalanine by ruthenium oxidation of a 3-amino-1,2-diol generated via coupling of an amine, and α-hydroxyaldehyde.
- β- and γ-allenols via metal-catalyzed cyclization. Allenols are used as a key precursor for the preparation of enantiopure dihydropyrans and tetrahydrooxepines.
- Isopropylidene D-glyceraldehyde intermediate, which controls the chirality in the total synthesis of prostaglandins (PGE1).
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Highly stereocontrolled one-step synthesis of anti-β-amino alcohols from organoboronic acids, amines, and α-hydroxy aldehydes
Petasis NA and Zavialov IA
Journal of the American Chemical Society, 120(45), 11798-11799 (1998)
Chiral synthesis of prostaglandins (PGE1) from D-glyceraldehyde
Stork G and Takahashi T
Journal of the American Chemical Society, 99(4), 1275-1276 (1977)
Takayoshi Wakagi et al.
PloS one, 11(1), e0147333-e0147333 (2016-01-26)
Archaea use glycolytic pathways distinct from those found in bacteria and eukaryotes, where unique enzymes catalyze each reaction step. In this study, we isolated three isozymes of glyceraldehyde oxidoreductase (GAOR1, GAOR2 and GAOR3) from the thermoacidophilic archaeon Sulfolobus tokodaii. GAOR1-3
Katarzyna Lechowicz et al.
International journal of molecular sciences, 21(16) (2020-08-13)
Lolium multiflorum/Festuca arundinacea introgression forms have been proved several times to be good models to identify key components of grass metabolism involved in the mechanisms of tolerance to water deficit. Here, for the first time, a relationship between photosynthetic and
Metal-Catalyzed Cyclization of β-and γ-Allenols Derived from d-Glyceraldehyde- Synthesis of Enantiopure Dihydropyrans and Tetrahydrooxepines: An Experimental and Theoretical Study
Alcaide BL
Chemistry?A European Journal, 15(36), 9127-9138 (2009)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica