About This Item
Produtos recomendados
Nível de qualidade
Ensaio
97%
pf
176 °C (lit.)
grupo funcional
carboxylic acid
thioether
cadeia de caracteres SMILES
OC(=O)C1NCCS1
InChI
1S/C4H7NO2S/c6-4(7)3-5-1-2-8-3/h3,5H,1-2H2,(H,6,7)
chave InChI
ULSZVNJBVJWEJE-UHFFFAOYSA-N
Descrição geral
Aplicação
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
Proline analogues are promising candidates for tuning the biological, pharmaceutical, or physicochemical properties of naturally occuring, as well as de novo designed, linear, and, cyclic peptides.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica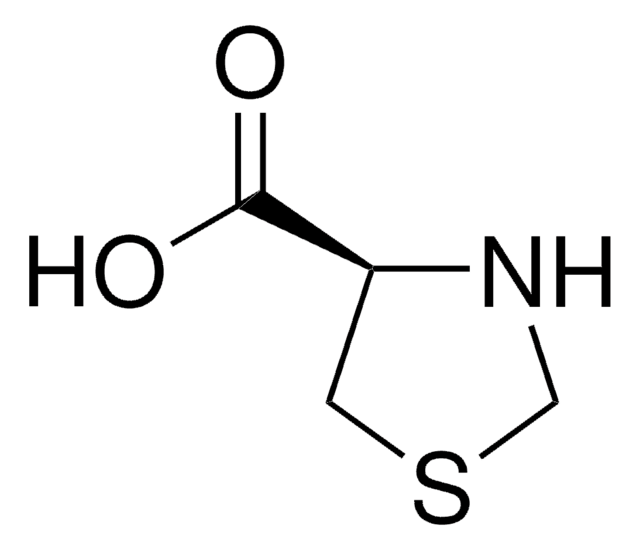

![2-[2-(Dimethylamino)ethoxy]ethanol 98%](/deepweb/assets/sigmaaldrich/product/structures/194/219/0fc5e46a-3a2e-48e4-b3b3-c63b710a5f99/640/0fc5e46a-3a2e-48e4-b3b3-c63b710a5f99.png)
![3,7-Diacetyl-1,3,7-triaza-5-phosphabicyclo[3.3.1]nonane 97%](/deepweb/assets/sigmaaldrich/product/structures/198/979/42d0b946-b026-4831-b284-fcb0e91533d9/640/42d0b946-b026-4831-b284-fcb0e91533d9.png)