433101
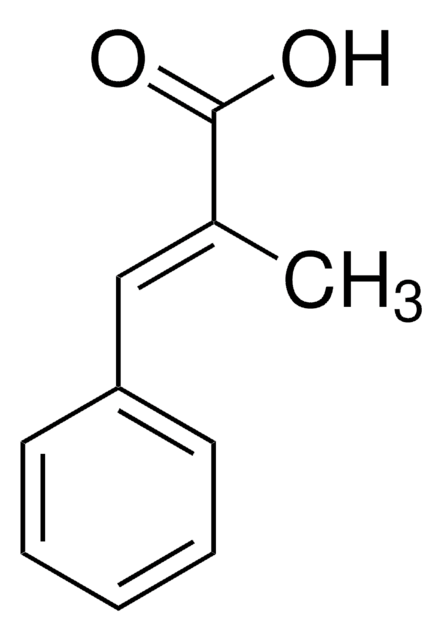
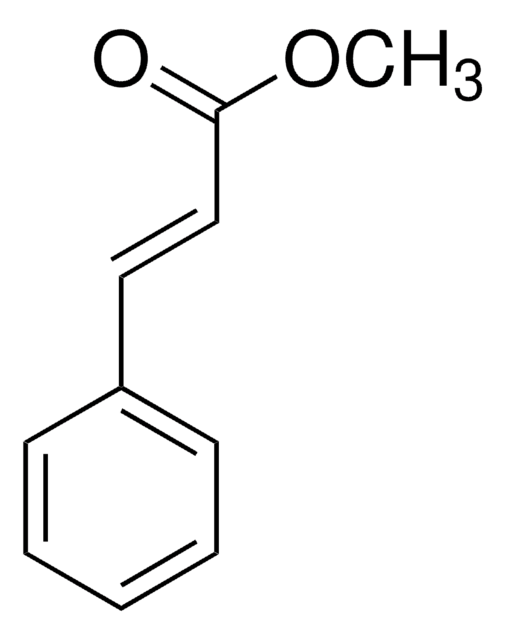
2-Methylcinnamic acid, predominantly trans
99%
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH3C6H4CH=CHCO2H
Número CAS:
Peso molecular:
162.19
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
99%
pf
174-176 °C (lit.)
grupo funcional
carboxylic acid
cadeia de caracteres SMILES
Cc1ccccc1\C=C\C(O)=O
InChI
1S/C10H10O2/c1-8-4-2-3-5-9(8)6-7-10(11)12/h2-7H,1H3,(H,11,12)/b7-6+
chave InChI
RSWBWHPZXKLUEX-VOTSOKGWSA-N
Descrição geral
2-Methylcinnamic acid has been reported to exhibit strong anti-fungal activity against white-rot fungus Lenzites betulina and brown-rot fungus Laetiporus sulphureus. Hydrogenation of 2-methylcinnamic acid using Walphos ligands and their biferrocene analogs has been studied.
Aplicação
2-Methylcinnamic acid may be used as starting reagent for the total synthesis of the cytotoxic alkaloid, 22-hydroxyacuminatine and for the preparation of (E)-2-methylcinnamic acid i-butylammonium salt.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Xiangshu Xiao et al.
Journal of medicinal chemistry, 49(4), 1408-1412 (2006-02-17)
A total synthesis of 22-hydroxyacuminatine, a cytotoxic alkaloid isolated from Camptotheca acuminata, is reported. The key step in the synthesis involves the reaction of 2,3-dihydro-1H-pyrrolo[3,4-b]quinoline with a brominated phthalide to generate a substituted pentacyclic 12H-5,11a-diazadibenzo[b,h]fluoren-11-one intermediate. Despite its structural resemblance
Sen-Sung Cheng et al.
Bioresource technology, 99(11), 5145-5149 (2007-10-20)
In this study, the antifungal activities of cinnamaldehyde and eugenol congeners against white-rot fungus Lenzites betulina and brown-rot fungus Laetiporus sulphureus were evaluated and the relationships between the antifungal activity and the chemical structures were also examined. Results from antifungal
Martin E Fox et al.
The Journal of organic chemistry, 73(3), 775-784 (2007-10-24)
Four chiral diphosphine ligands consisting of bis(2,5-diphenylphospholan-1-yl) groups connected by the sp(2) carbon linkers 2,3-quinoxaline ((S,S)-Ph-Quinox), 2,3-pyrazine ((S,S)-Ph-Pyrazine), maleic anhydride ((S,S)-Ph-MalPhos), and 1,1'-ferrocene ((S,S)-Ph-5-Fc) were synthesized, and their cationic [rhodium(I)(COD)] complexes were prepared. These complexes were tested in asymmetric hydrogenation
Afrooz Zirakzadeh et al.
Organometallics, 33(8), 1945-1952 (2014-05-06)
Two representative Walphos analogues with an achiral 2,2″-biferrocenediyl backbone were synthesized. These diphosphine ligands were tested in the rhodium-catalyzed asymmetric hydrogenation of several alkenes and in the ruthenium-catalyzed hydrogenation of two ketones. The results were compared with those previously obtained
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica