433063
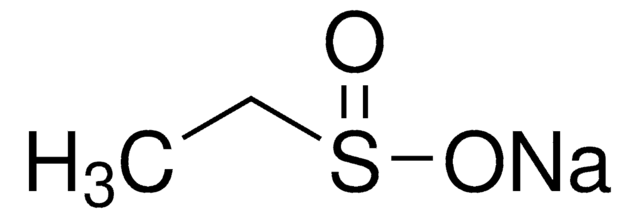
Sodium methanesulfinate
technical grade, 85%
Sinônimo(s):
Methanesulfinic acid sodium salt
Faça loginpara ver os preços organizacionais e de contrato
About This Item
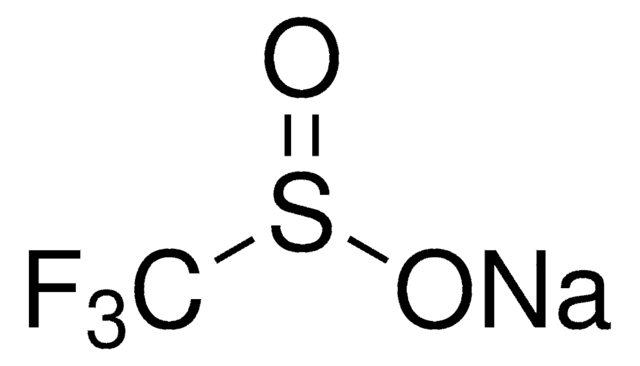
Fórmula linear:
CH3SO2Na
Número CAS:
Peso molecular:
102.09
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
grau
technical grade
Nível de qualidade
Ensaio
85%
pf
222-226 °C (dec.) (lit.)
grupo funcional
sulfinic acid
cadeia de caracteres SMILES
[Na+].CS([O-])=O
InChI
1S/CH4O2S.Na/c1-4(2)3;/h1H3,(H,2,3);/q;+1/p-1
chave InChI
LYPGDCWPTHTUDO-UHFFFAOYSA-M
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Sodium methanesulfinate is an aliphatic sodium sulfinate. Conjugate addition of sodium methanesulfinate to vinyl heterocycles has been described. Cross-coupling reaction between aryl boronic acid and sodium methanesulfinate has been studied. Its stock solution was prepared from methanesulfonic acid by adding one equivalent of sodium hydroxide and diluting it to 4M.
Palavra indicadora
Warning
Frases de perigo
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Metal-Free Direct Construction of Sulfonamides via Iodine-Mediated Coupling Reaction of Sodium Sulfinates and Amines at Room Temperature.
Wei W, et al.
Advanced Synthesis & Catalysis, 357(5), 987-992 (2015)
J B Smith et al.
Free radical research communications, 8(2), 101-106 (1990-01-01)
Iron bound to certain chelators is known to promote the conversion of superoxide radicals (O2-) to hydroxyl radicals (HO.) by the superoxide-driven Fenton reaction. The production of HO. by various iron chelates was studied using the reaction of dimethyl sulfoxide
M G Steiner et al.
Archives of biochemistry and biophysics, 278(2), 478-481 (1990-05-01)
This investigation was conducted to validate the use of dimethyl sulfoxide (DMSO) as a quantitative molecular probe for the generation of hydroxyl radicals (HO.) in aqueous systems. Reaction of HO. with DMSO produces methane sulfinic acid as a primary product
M G Steiner et al.
Free radical biology & medicine, 9(1), 67-77 (1990-01-01)
To quantitate the formation of hydroxyl radicals (HO.) in ischemia and reoxygenation, dimethyl sulfoxide (DMSO) was added to "trap" evolving HO. in normal, in ischemic, and in ischemic and reoxygenated rat kidney slices, incubated in short-term organ culture in vitro.
S Fukui et al.
Journal of chromatography, 630(1-2), 187-193 (1993-02-05)
For the determination of hydroxyl radicals, dimethyl sulphoxide was used as a molecular probe and the methanesulphinic acid produced was determined by high-performance liquid chromatography of its Fast Yellow GC salt derivative. The results for hydroxyl radicals formed using the
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica

![1,8-Diazabiciclo[5,4,0]undec-7-eno 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)