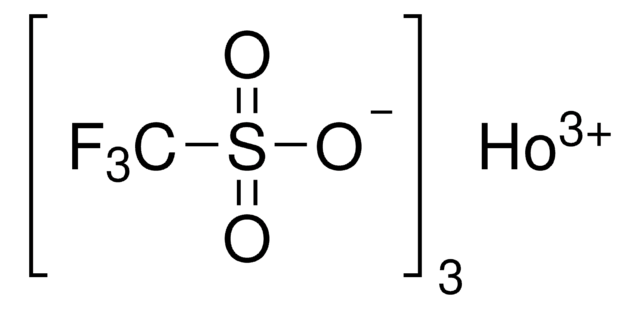
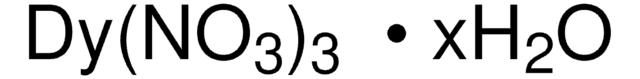425664
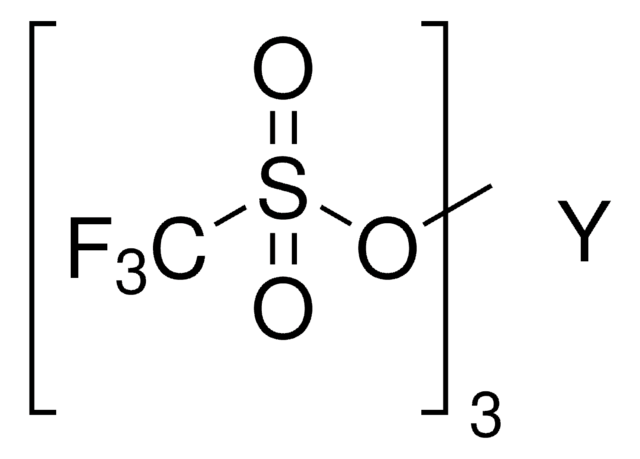
Dysprosium(III) trifluoromethanesulfonate
98%
Sinônimo(s):
Dysprosium(III) triflate, Tris(triflato)dysprosium
About This Item
Produtos recomendados
Ensaio
98%
adequação da reação
core: dysprosium
reagent type: catalyst
reaction type: Ring-Opening Polymerization
cadeia de caracteres SMILES
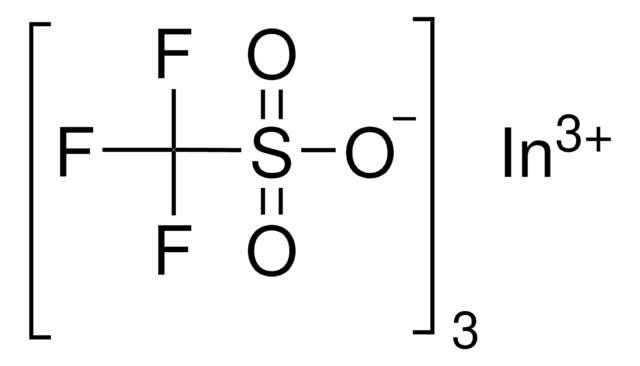
[Dy+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Dy/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
chave InChI
XSVCYDUEICANRJ-UHFFFAOYSA-K
Categorias relacionadas
Descrição geral
Aplicação
- Aza-Piancatelli rearrangement
- Friedel-Crafts alkylation
- Ring-opening polymerization reactions
- Microwave-assisted Kabachnik-Fields condensation
- Cycloaddition reactions (Lewis-acid catalyst)
- Fries rearrangement
- Enantioselective glyoxalate-ene reactions
- Aldol reaction of silyl enol ethers with aldehydes.
- As an effective catalyst for electrophilic substitution reactions of indoles with imines.
- As catalyst for the synthesis of 4-aminocyclopentenones and functionalized azaspirocycles, via intramolecular aza-Piancatelli rearrangement.
- As new curing initiator to study the curing of diglycidyl ether of bisphenol-A (DGEBA).
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica