About This Item
Fórmula empírica (Notação de Hill):
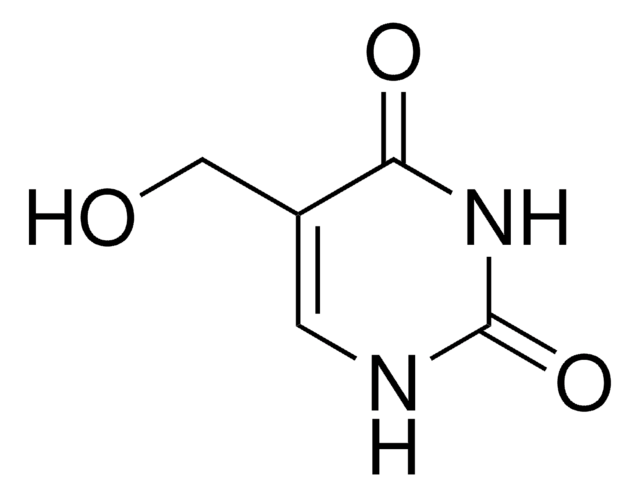
C5H4N2O3
Número CAS:
Peso molecular:
140.10
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
powder
pf
>300 °C (dec.) (lit.)
grupo funcional
aldehyde
cadeia de caracteres SMILES
O=CC1=CNC(=O)NC1=O
InChI
1S/C5H4N2O3/c8-2-3-1-6-5(10)7-4(3)9/h1-2H,(H2,6,7,9,10)
chave InChI
OHAMXGZMZZWRCA-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
5-Formyluracil may be used for the preparation of covalently linked base with 5-aminocytosine pair via Schiff base formation.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Q M Zhang et al.
International journal of radiation biology, 79(5), 341-349 (2003-08-29)
5-Formyluracil (5-foU) is a potentially mutagenic lesion of thymine produced in DNA by ionizing radiation and various chemical oxidants. The present authors reported previously that MutM, Nth and Nei in Escherichia coli removed 5-foU from DNA. The present study identified
Gustavo Portalone et al.
Acta crystallographica. Section C, Crystal structure communications, 63(Pt 11), o650-o654 (2007-11-09)
The asymmetric unit of the amino-oxo tautomer of 5-formyluracil (systematic name: 2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carbaldehyde), C(5)H(4)N(2)O(3), comprises one planar amino-oxo tautomer, as every atom in the structure lies on a crystallographic mirror plane. At variance with all the previously reported small-molecule crystal structures
Monica Baldini et al.
Inorganic chemistry, 42(6), 2049-2055 (2003-03-18)
Two new 5-formyluracil thiosemicarbazone (H(3)ut) derivatives, Me-H(3)ut (1) and Me(2)-H(3)ut (2), were synthesized by reacting thiosemicarbazides, mono- and dimethylated on the aminic nitrogen, with 5-formyluracil and were subsequently characterized. These ligands, treated with copper chloride and nitrate, afforded three complexes:
Chikara Dohno et al.
Journal of the American Chemical Society, 127(47), 16681-16684 (2005-11-25)
We here present a novel covalently linked base pair via Schiff base formation between 5-formyluracil (fU) and 5-aminocytosine (AmC). Formation of the Schiff base linkage proceeds reversibly and does not require any additives. The cross-linked DNA is very stable under
E J Privat et al.
Mutation research, 354(2), 151-156 (1996-07-22)
5-Formyluracil is a mutagenic base formed in DNA by oxidation of the thymine methyl group. Whereas the thymine methyl group is electron donating, the formyl group is electron withdrawing, predicting increased ionization of the N-3 imino proton under physiological conditions.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica