400424
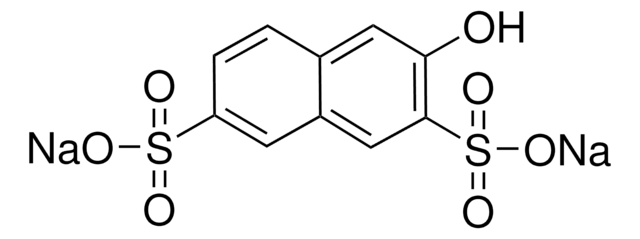
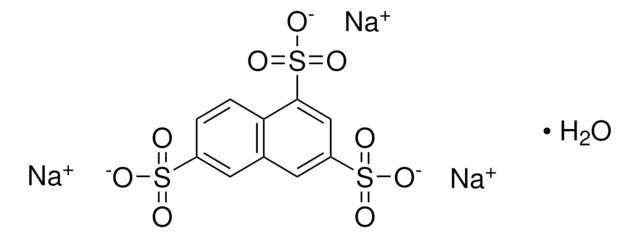
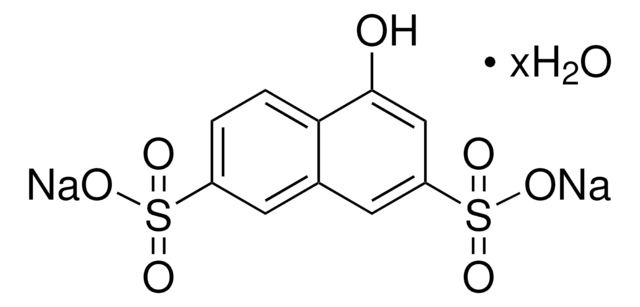
6-Hydroxy-2-naphthalenesulfonic acid sodium salt hydrate
technical grade
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
HOC10H6SO3Na · xH2O
Peso molecular:
246.21 (anhydrous basis)
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
grau
technical grade
Nível de qualidade
Formulário
solid
pf
>300 °C (lit.)
grupo funcional
sulfonic acid
cadeia de caracteres SMILES
O.[Na+].Oc1ccc2cc(ccc2c1)S([O-])(=O)=O
InChI
1S/C10H8O4S.Na.H2O/c11-9-3-1-8-6-10(15(12,13)14)4-2-7(8)5-9;;/h1-6,11H,(H,12,13,14);;1H2/q;+1;/p-1
chave InChI
QHVRFNCHOMKXQP-UHFFFAOYSA-M
Descrição geral
Sodium salt of 6-hydroxy-2-naphthalenesulfonic acid is also referred as Schaeffer′s salt.
Aplicação
6-Hydroxy-2-naphthalenesulfonic acid sodium salt hydrate may be used in the following studies:
- As precursors during the agar-plate screening test.
- Preparation of methoxy-substituted naphthalenesulfonyl chloride.
- Synthesis of FD&C Red No. 40, a synthetic water-soluble color that is permitted for coloring foods, drugs and cosmetics in the U.S.A.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Anamarija Zega et al.
Bioorganic & medicinal chemistry letters, 14(6), 1563-1567 (2004-03-10)
A series of azaphenylalanine derivatives were investigated as novel thrombin inhibitors based on the prodrug principle. By systematic structural modifications we have identified optimal groups for this series that led us to potent inhibitors of thrombin incorporating the benzamidine fragment
N Richfield-Fratz et al.
Journal of chromatography, 467(1), 167-176 (1989-04-21)
The unsulfonated aromatic amine 4-nitro-p-cresidine (2-methoxy-5-methyl-4-nitrobenzenamine) was identified as an impurity in the regulated color additive FD&C Red. No. 40. The compound was isolated from the water-soluble color by extraction with chloroform, followed by transfer of the free amines to
Jolanta Polak et al.
Microbial cell factories, 9, 51-51 (2010-07-06)
Chemical methods of producing dyes involve extreme temperatures and unsafe toxic compounds. Application of oxidizing enzymes obtained from fungal species, for example laccase, is an alternative to chemical synthesis of dyes. Laccase can be replaced by fungal biomass acting as
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica