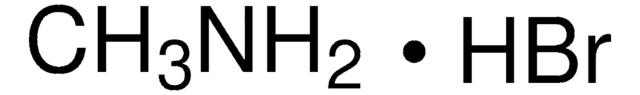398853
Lead(II) bromide
99.999% trace metals basis
Sinônimo(s):
Lead dibromide
About This Item
Produtos recomendados
grau
for analytical purposes
Ensaio
99.999% trace metals basis
Formulário
powder
adequação da reação
core: lead
Impurezas
≤15.0 ppm Trace Metal Analysis
p.e.
892 °C (lit.)
pf
371 °C (lit.)
densidade
6.66 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
Br[PbH2]Br
InChI
1S/2BrH.Pb/h2*1H;/q;;+2/p-2
chave InChI
ZASWJUOMEGBQCQ-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- To prepare an electrolyte for a high performance all-solid-state bromide-ion battery.
- As a precursor to prepare organic–inorganic hybrid perovskite materials for solar cells and light emitting devices (LEDs).
- As a starting material to prepare photocatalytic CsPbBr3@SiO2 composites with good water stability.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1A - STOT RE 2
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Since the first report of the low-cost dye-sensitized solar cell (DSSC) in 1991 by Gratzel and his coworker,1 dye-sensitized solar cells (DSSC) has been regarded as one of the most promising photovoltaic technologies because of their transparent and colorful characteristics, as well as low cost.
The past several decades have seen major advancements in the synthesis of metal nanomaterials. Most recently, controlled synthesis has become versatile enough to regulate the exact number of atoms and ligands of very small metal nanoparticles, referred to as “clusters”.
Next generation solar cells have the potential to achieve conversion efficiencies beyond the Shockley-Queisser (S-Q) limit while also significantly lowering production costs.
Dr. Perini and Professor Correa-Baena discuss the latest research and effort to obtain higher performance and stability of perovskite materials.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica