About This Item
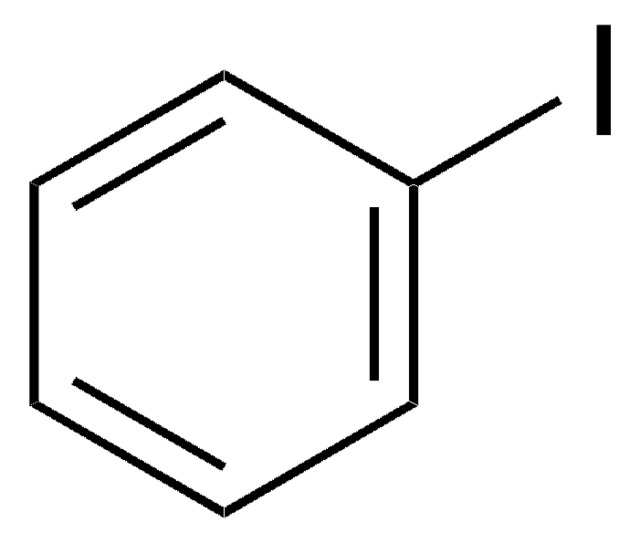
Fórmula linear:
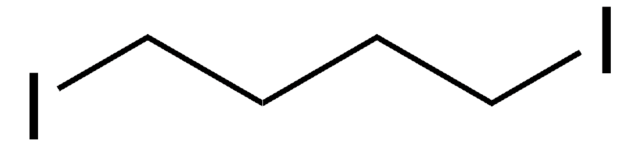
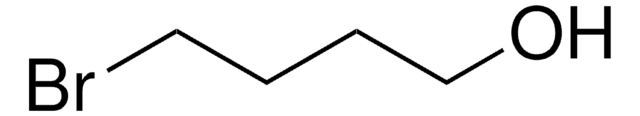
I(CH2)4Cl
Número CAS:
Peso molecular:
218.46
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
liquid
contém
copper as stabilizer
índice de refração
n20/D 1.54 (lit.)
p.e.
88-89 °C/19 mmHg (lit.)
densidade
1.785 g/mL at 25 °C (lit.)
grupo funcional
chloro
iodo
cadeia de caracteres SMILES
ClCCCCI
InChI
1S/C4H8ClI/c5-3-1-2-4-6/h1-4H2
chave InChI
JXOSPTBRSOYXGC-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
1-Chloro-4-iodobutane is a halogenated hydrocarbon. It is an α,ω-dihaloalkane and undergoes electrogenerated Nickel(I) salen (N,N′-bis(salicylidene)ethylenediamine) catalyzed reduction to afford 1,8-dichlorooctane. Electrochemical reduction of 1-chloro-4-iodobutane at glassy carbon cathode has been investigated by cyclic voltammetry and controlled-potential electrolysis.
Aplicação
1-Chloro-4-iodobutane may be used in the following studies:
- Preparation of 6-hendecenoic acid.
- Catalytic asymmetric synthesis of levobupivacaine.
- Synthesis of alkaloids such as deoxyvasicinone, mackinazolinone.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
199.4 °F - closed cup
Ponto de fulgor (°C)
93 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Studies directed towards asymmetric synthesis of levobupivacaine.
Kumar S and Ramachandran U.
Tetrahedron Letters, 46(1), 19-21 (2005)
The synthesis of unsaturated fatty acids.
K AHMAD et al.
Journal of the American Chemical Society, 70(5), 1699-1699 (1948-05-01)
W Russell Bowman et al.
Organic & biomolecular chemistry, 5(1), 103-113 (2006-12-14)
Alkyl, aryl, heteroaryl and acyl radicals have been cyclised onto the 2-position of 3H-quinazolin-4-one. The side chains containing the radical precursors were attached to the nitrogen atom in the 3-position. The cyclisations take place by aromatic homolytic substitution hence retain
Electrochemical reduction of 1, 4-dihalobutanes at carbon cathodes in dimethylformamide.
Pritts WA and Peters DG.
Journal of Electroanalytical Chemistry, 380(1), 147-160 (1995)
Keivan Sadrerafi et al.
Drug design, development and therapy, 12, 987-995 (2018-05-08)
Our previous study indicated that carborane containing small-molecule 1-(hydroxymethyl)-7-(4'-(trans-3″-(3'″-pyridyl)acrylamido)butyl)-1,7-dicarbadodecaborane (hm-MC4-PPEA), was a potent inhibitor of nicotinamide phosphoribosyltransferase (Nampt). Nampt has been shown to be upregulated in most cancers and is a promising target for the treatment of many different types
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica